Iec 62304 Software Development Plan Template
Here are some of the images for Iec 62304 Software Development Plan Template that we found in our website database.

creation of an iec 62304 compliant software development plan 56yqyqkbo1
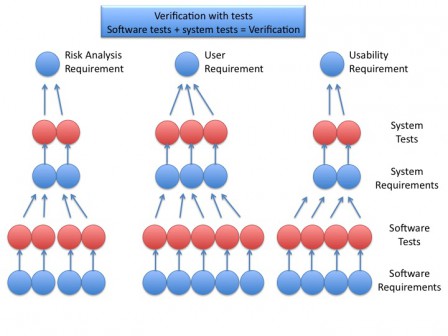
Iec 62304 Software Development Plan Template cubepriority
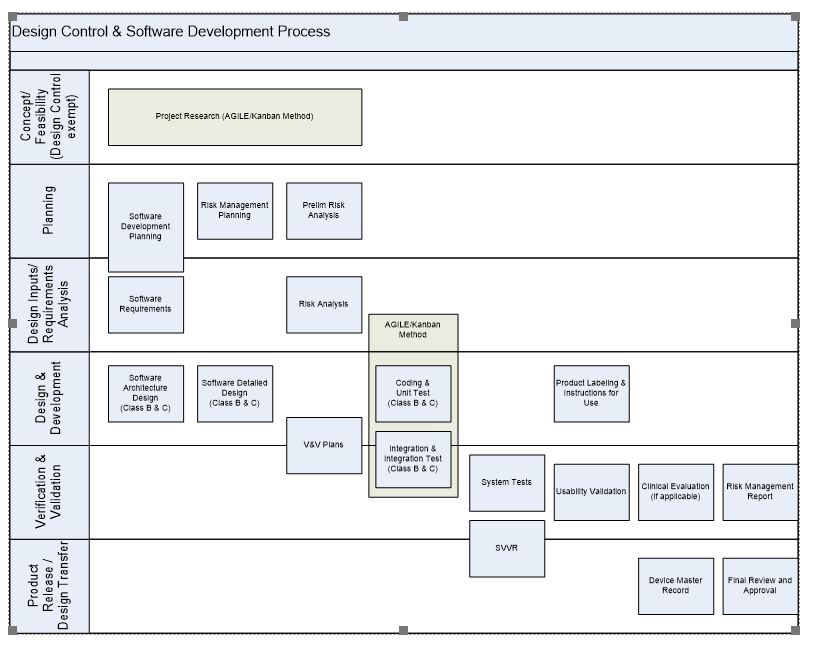
Iec 62304 Software Development Plan Template

Iec 62304 Software Development Plan Template
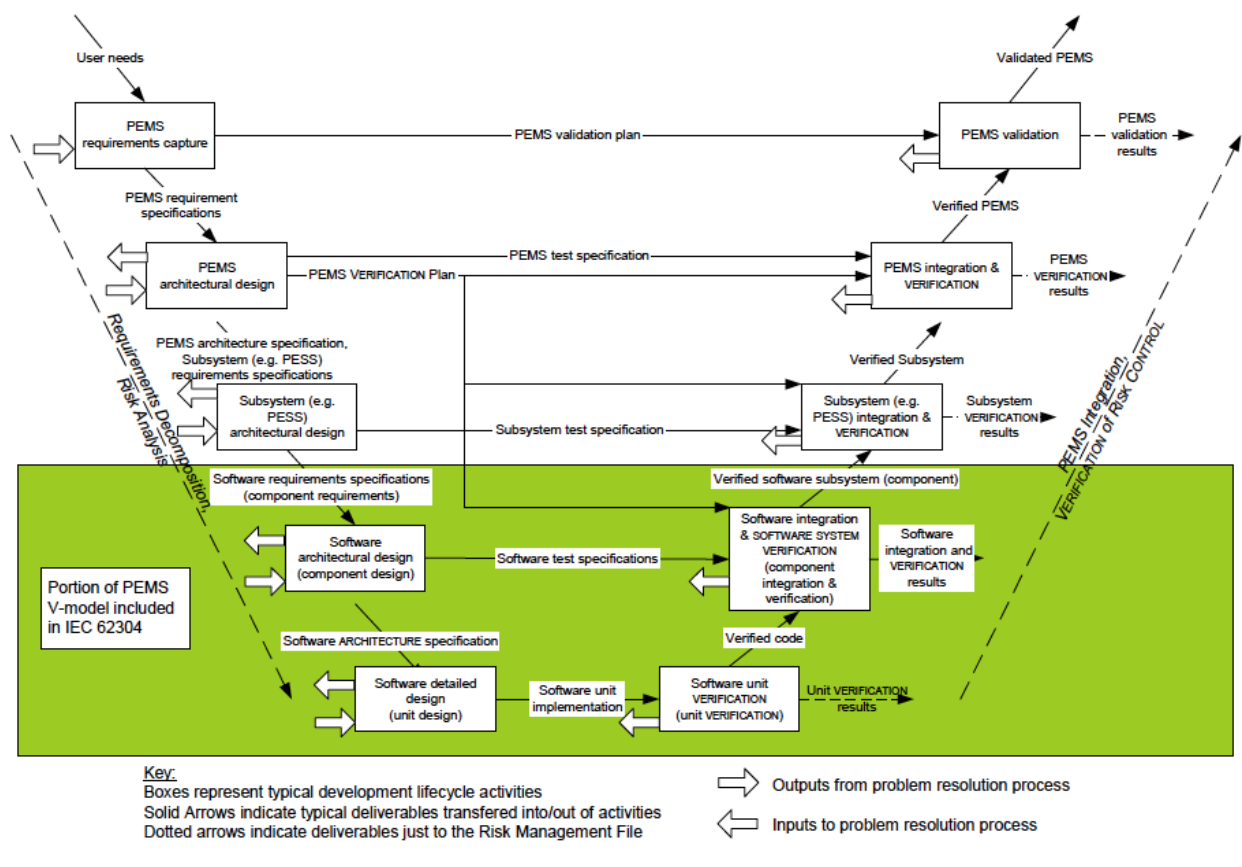
Iec 62304 Software Development Plan Template
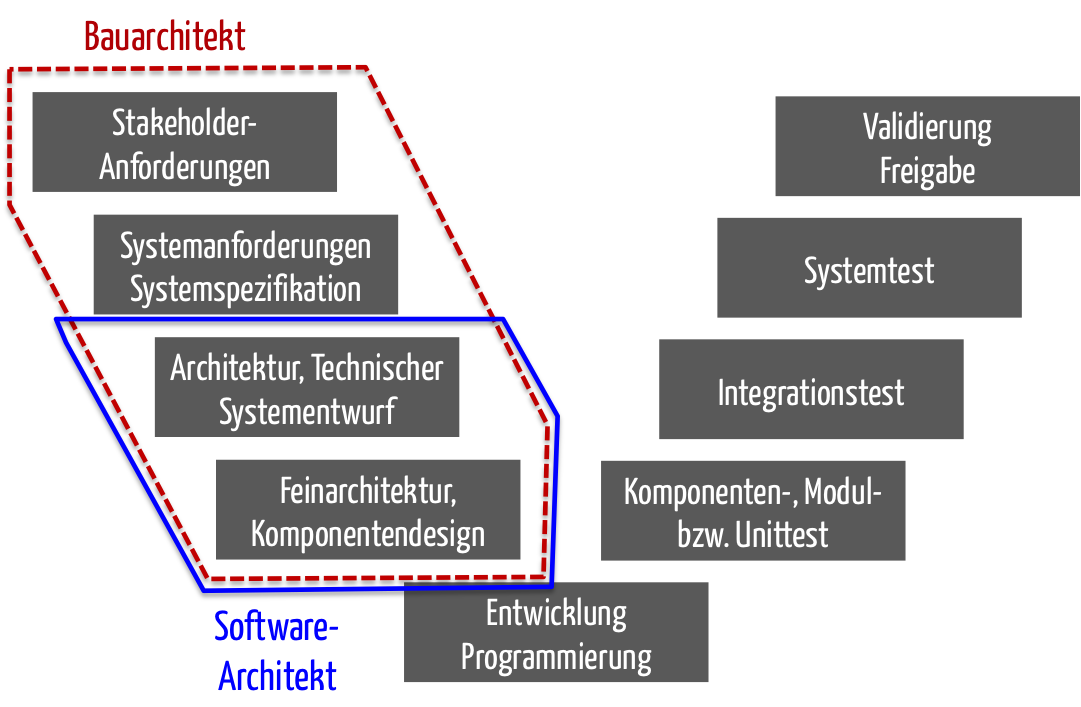
Iec 62304 Software Development Plan Template udlvirtual esad edu br

Iec 62304 Software Development Plan Template udlvirtual esad edu br
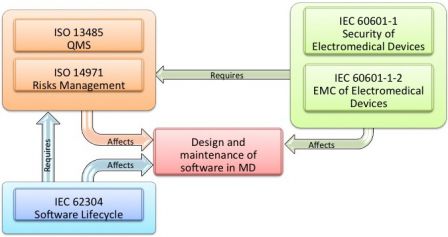
Iec 62304 Software Development Plan Template udlvirtual esad edu br
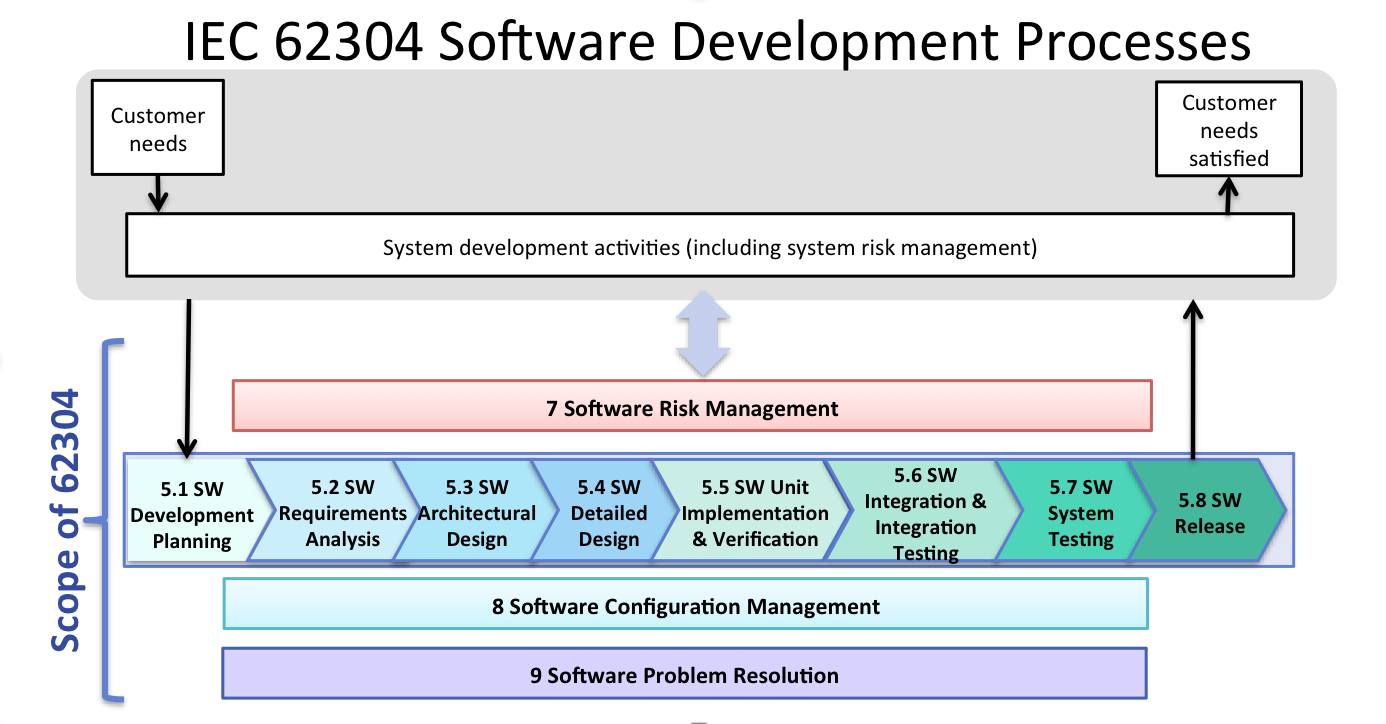
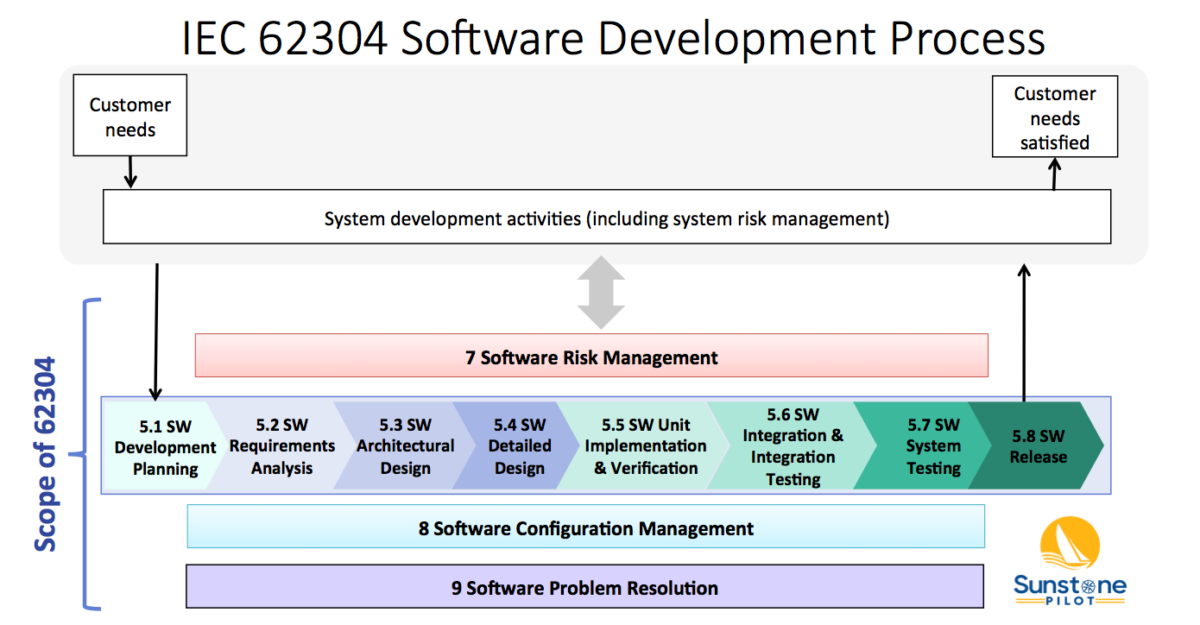
IEC 62304 Software Development Process Sunstone Pilot Inc

Iec 62304 Software Development Plan Template udlvirtual esad edu br

Iec 62304 Software Development Plan Template udlvirtual esad edu br
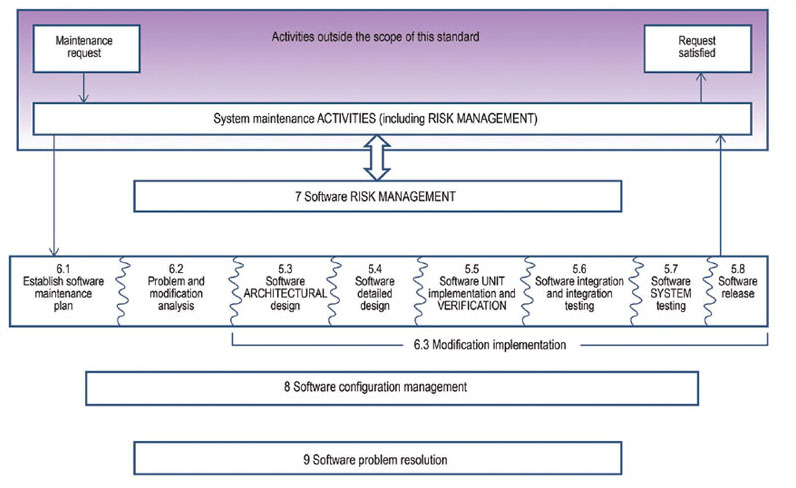
Iec 62304 Software Development Plan Template udlvirtual esad edu br
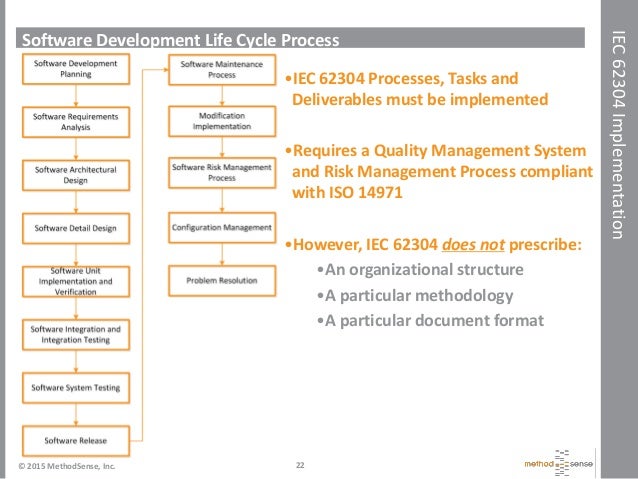
Iec 62304 Software Development Plan Template udlvirtual esad edu br
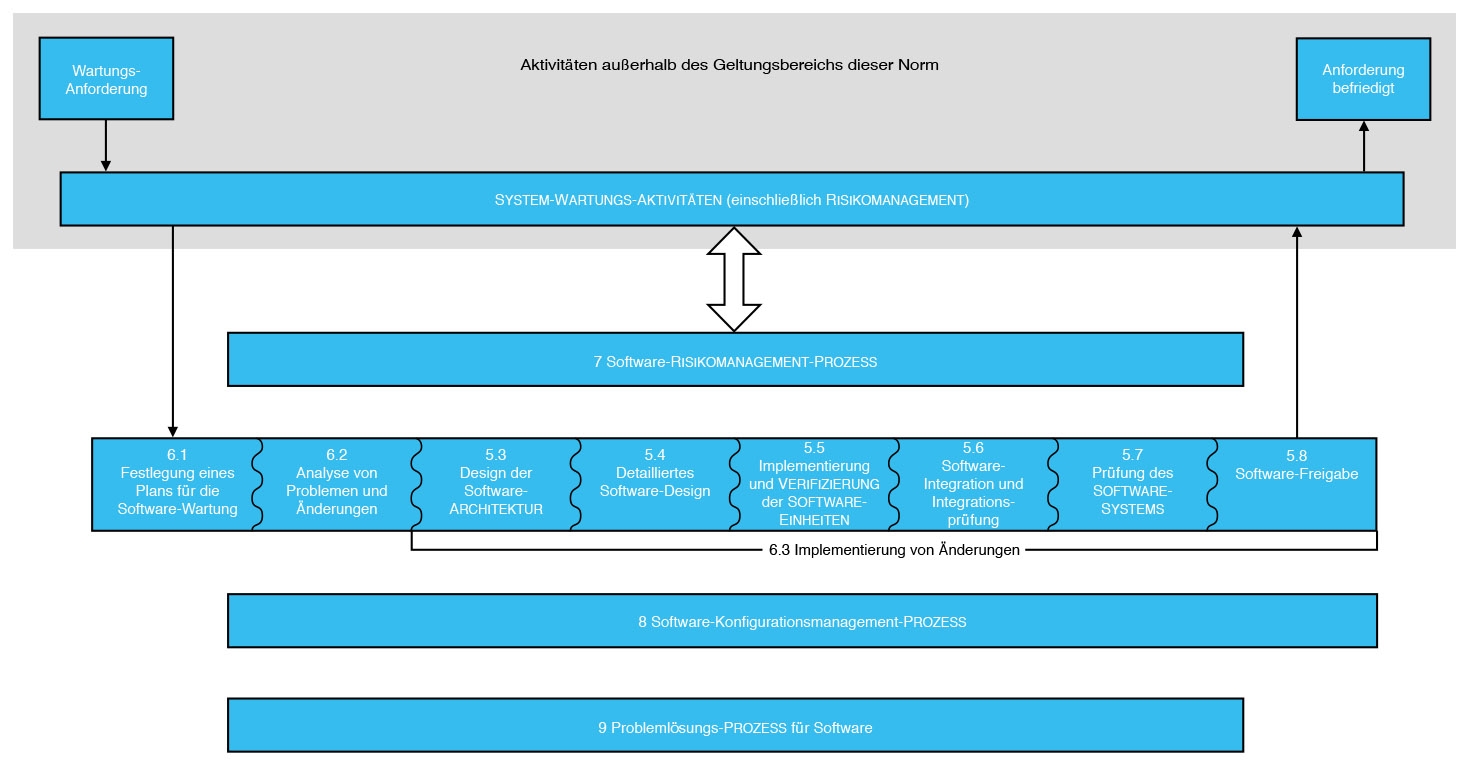
Iec 62304 Software Development Plan Template udlvirtual esad edu br
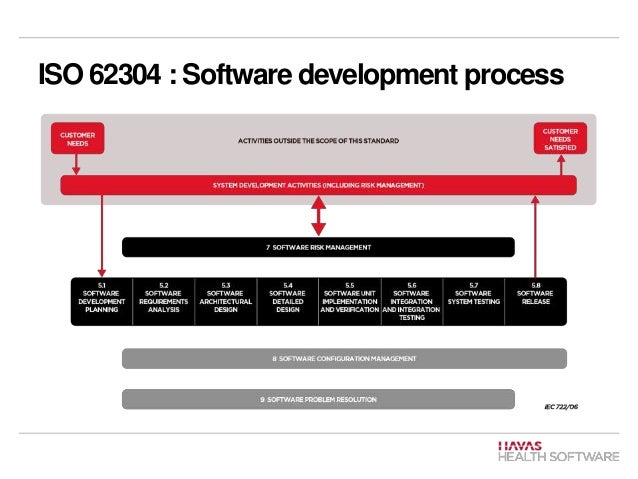
Iec 62304 Software Development Plan Template udlvirtual esad edu br

Iec 62304 Software Development Plan Template udlvirtual esad edu br
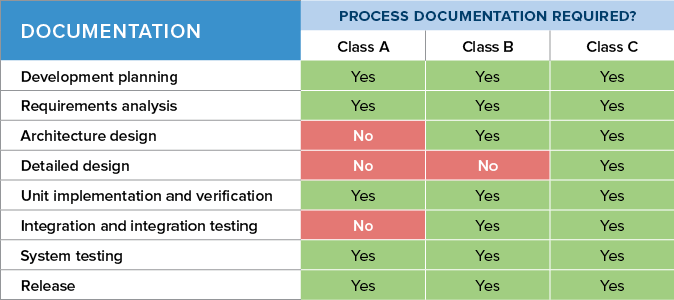
Iec 62304 Software Development Plan Template udlvirtual esad edu br

Iec 62304 Software Development Plan Template

Iec 62304 Software Development Plan Template

Software Development Plan Template MedicalDeviceHQ

IEC 62304 standard for medical device software development MATLAB
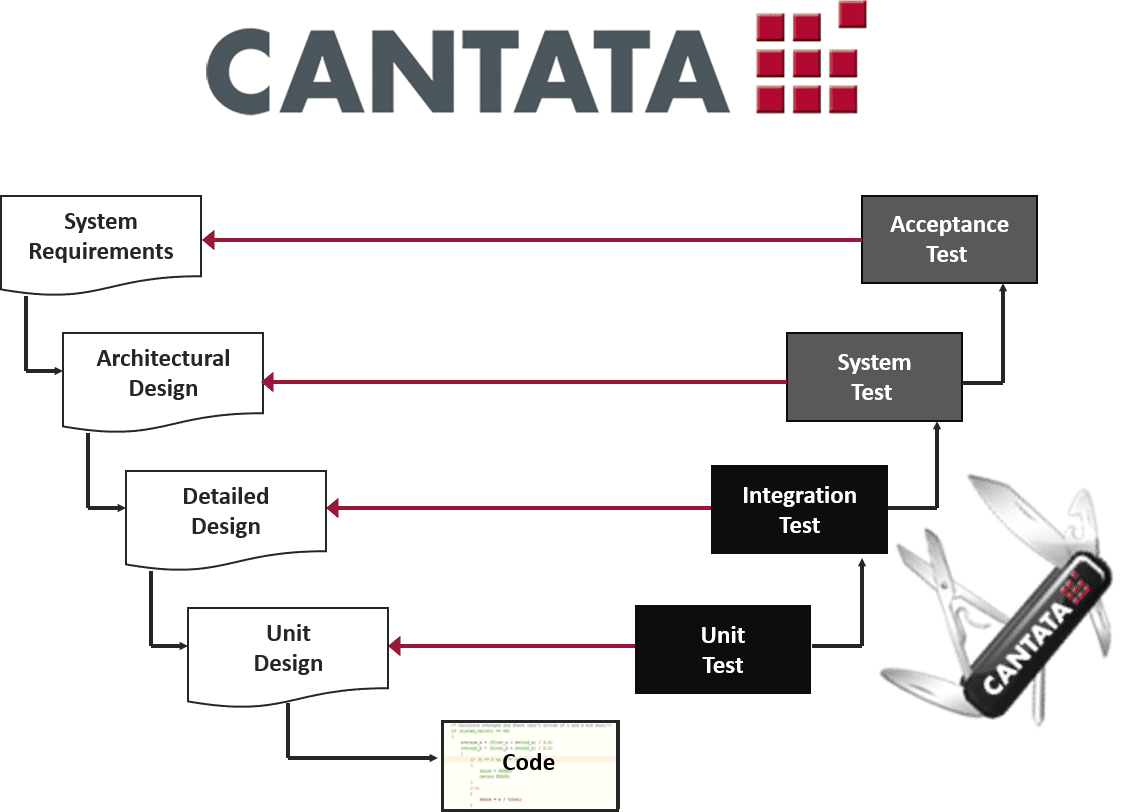
IEC 62304 FDA Software Validation Compliance QA Systems
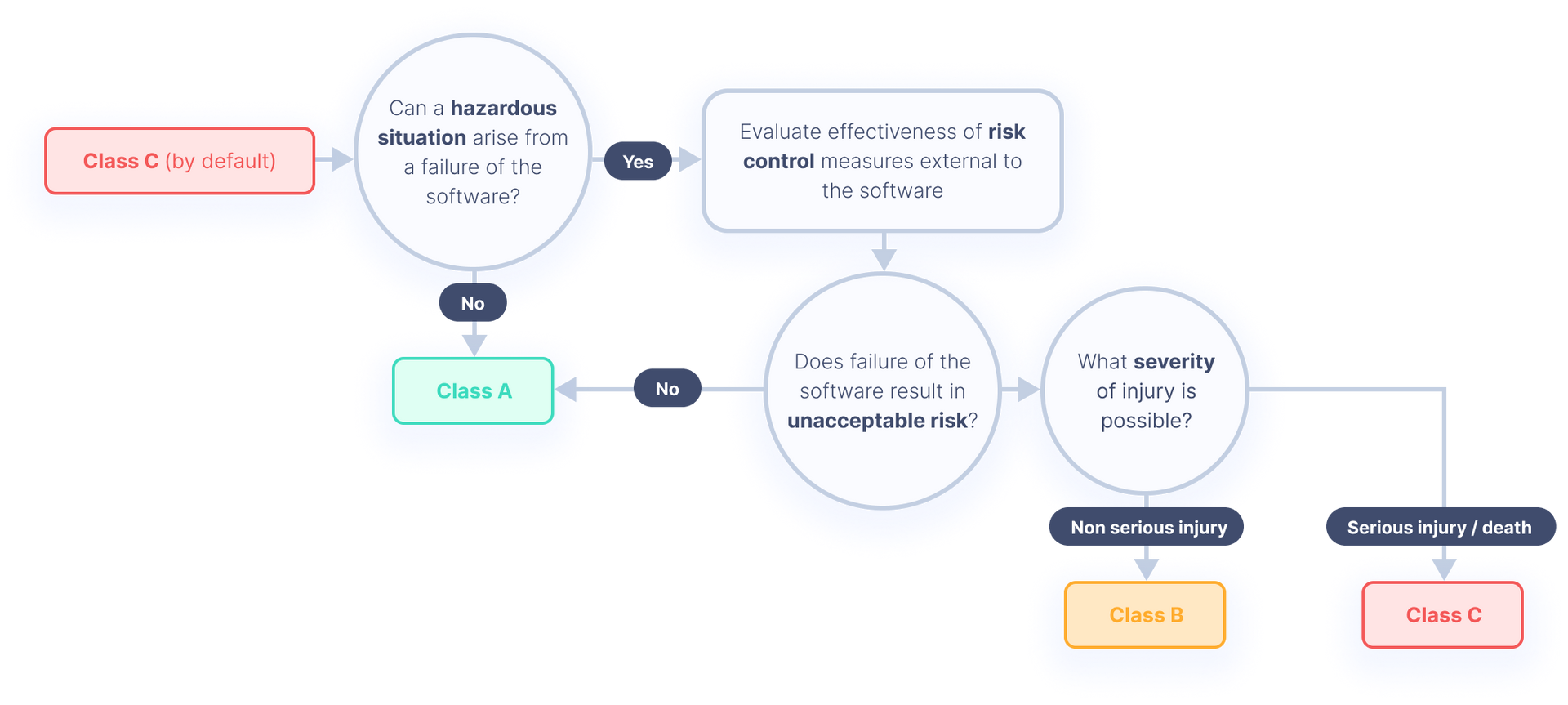
All You Need to Know About IEC 62304 Development for Medical Devices
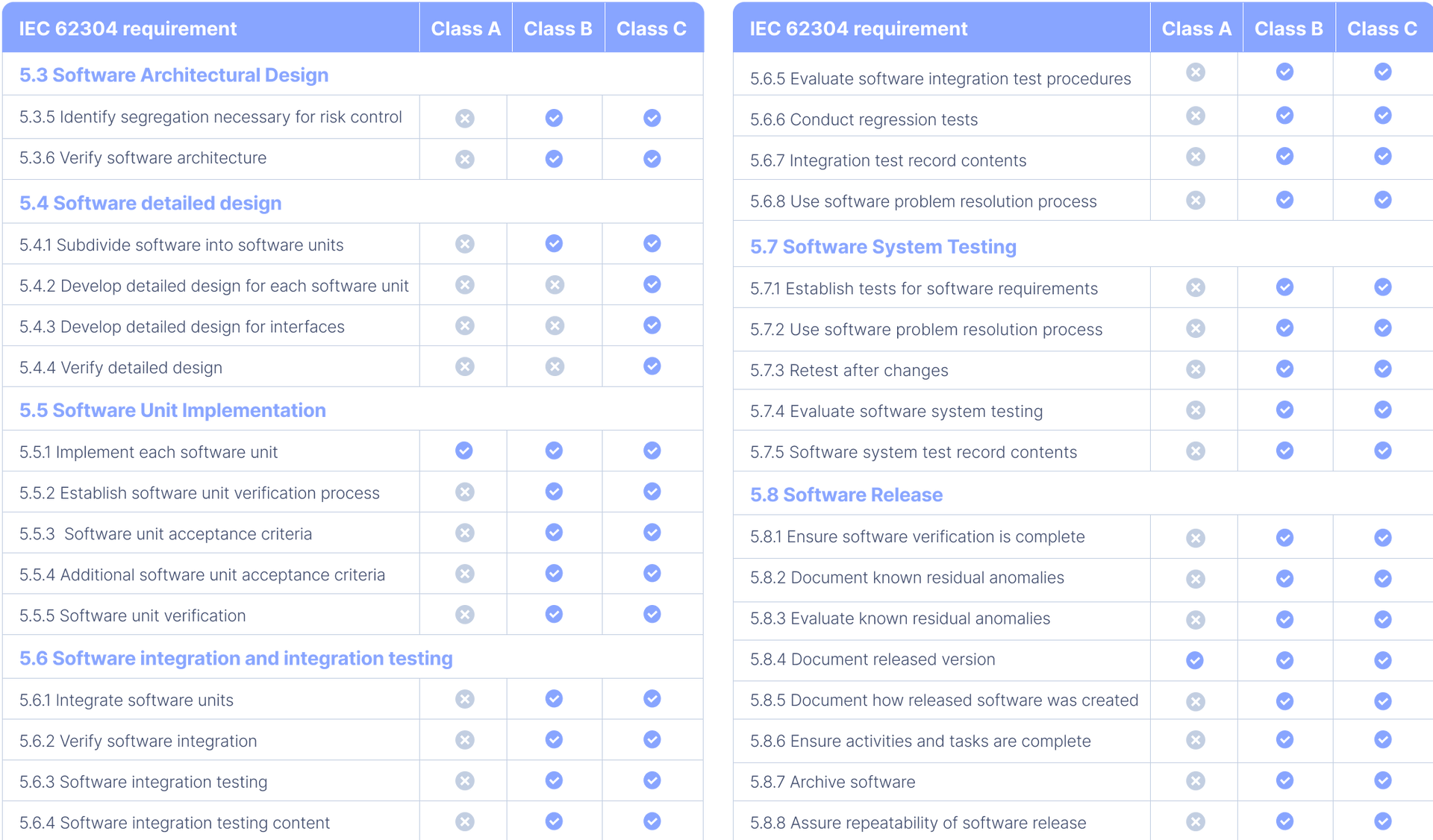
All You Need to Know About IEC 62304 Development for Medical Devices
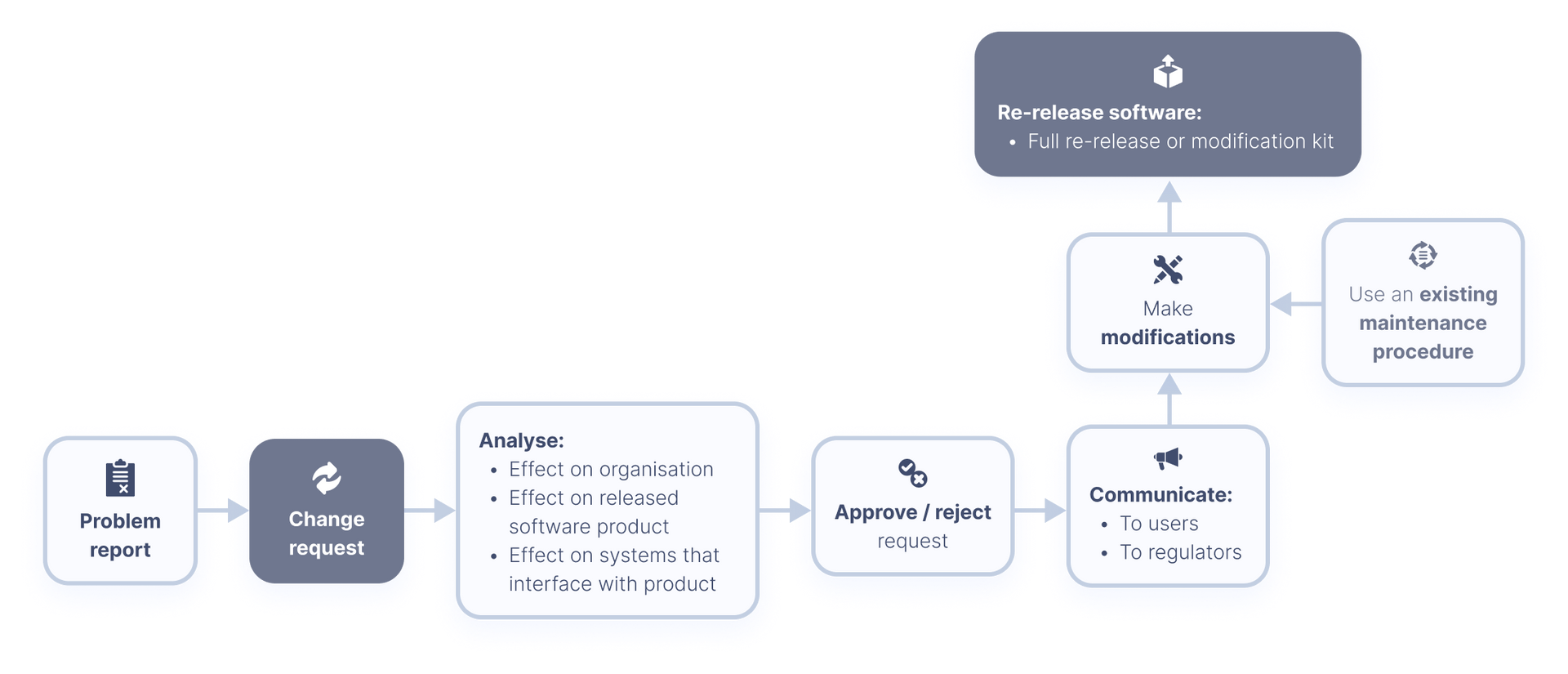
All You Need to Know About IEC 62304 Development for Medical Devices
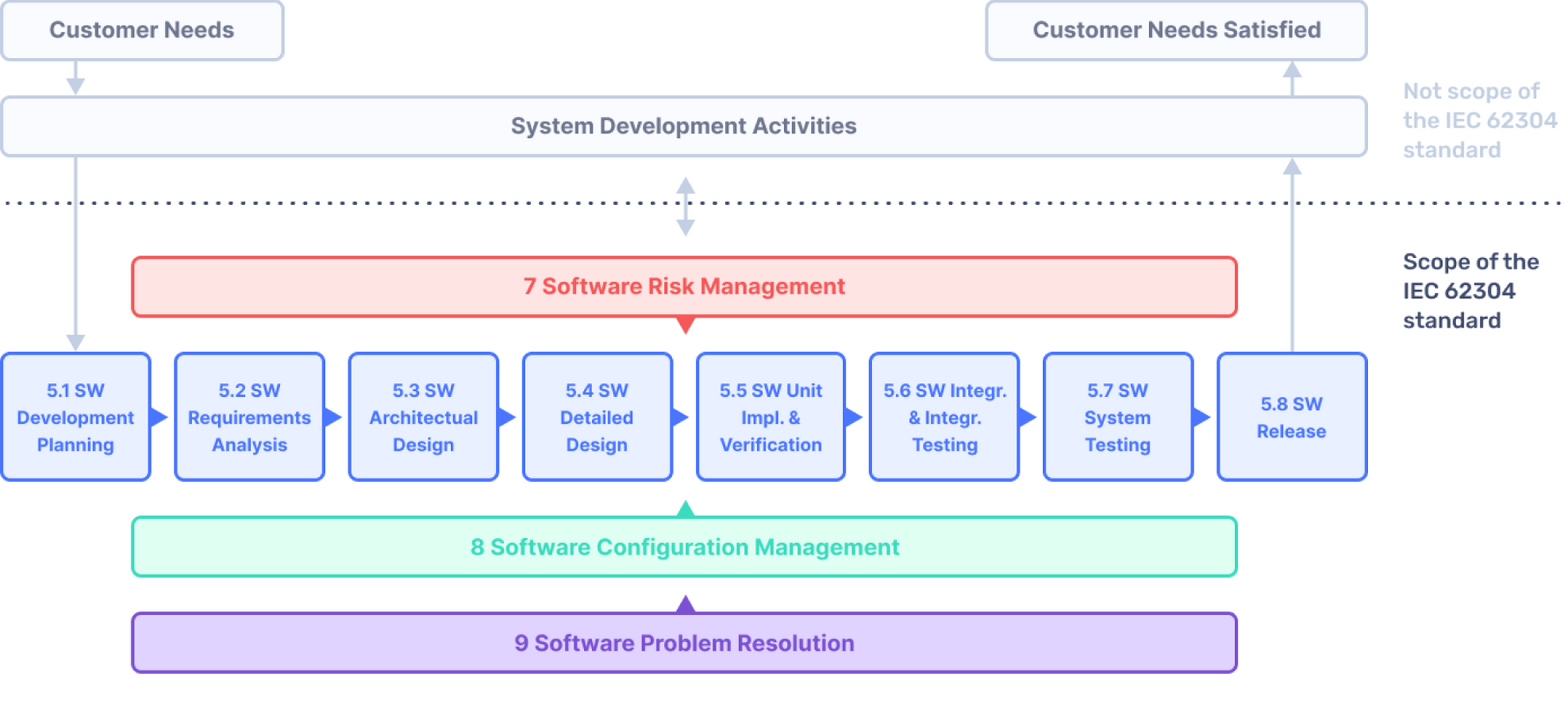
All You Need to Know About IEC 62304 Development for Medical Devices
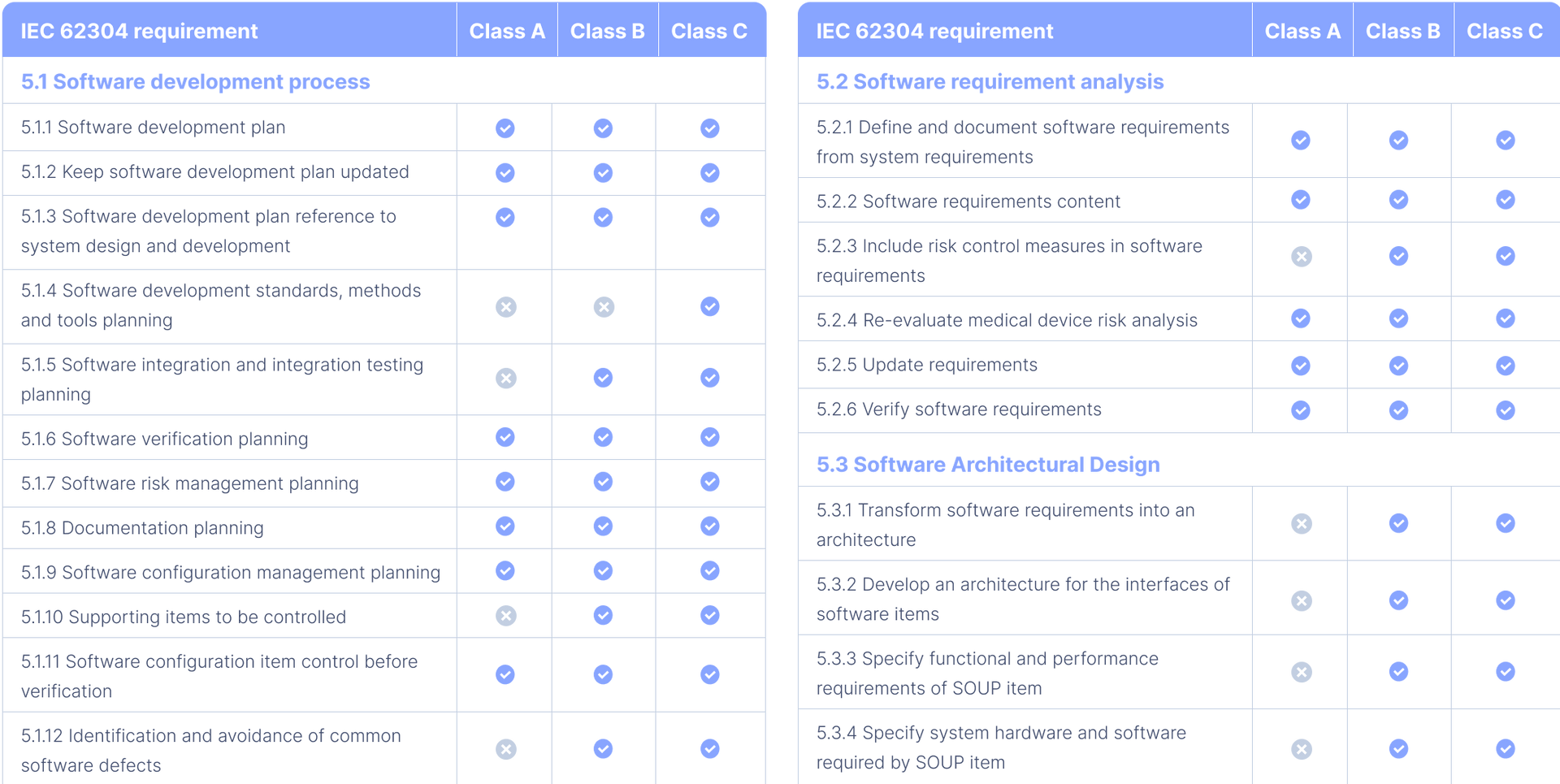
All You Need to Know About IEC 62304 Development for Medical Devices

All You Need to Know About IEC 62304 Development for Medical Devices
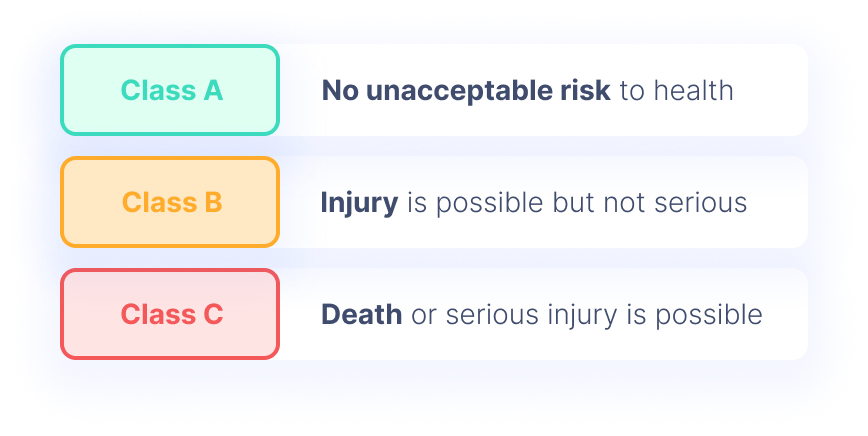
All You Need to Know About IEC 62304 Development for Medical Devices
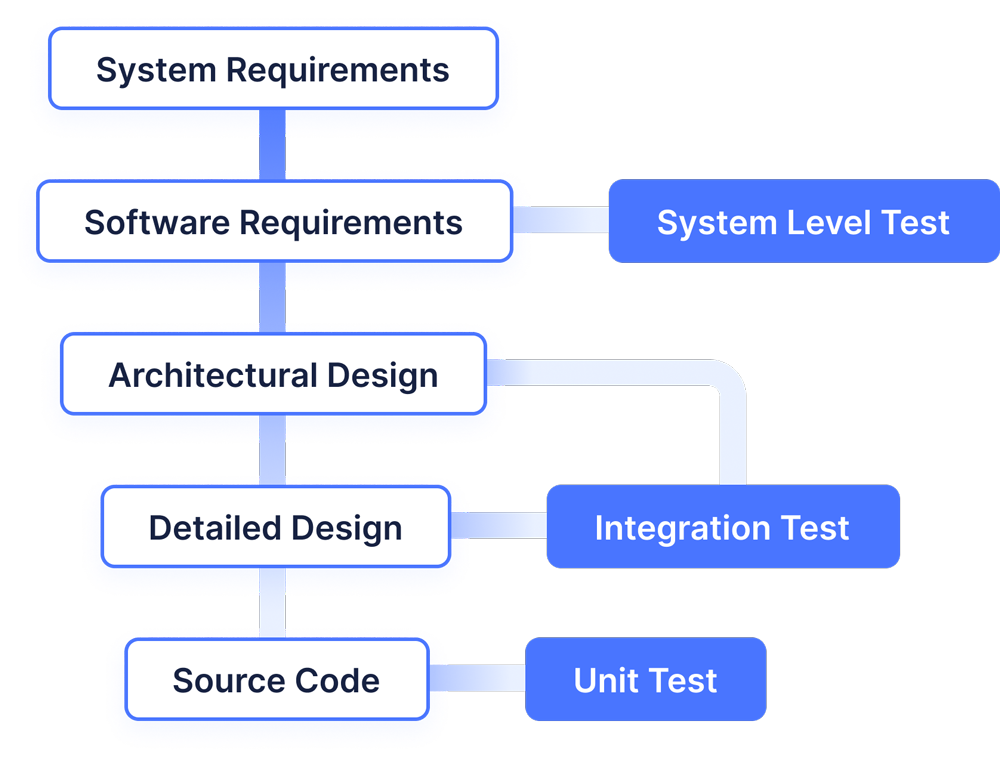
All You Need to Know About IEC 62304 Development for Medical Devices
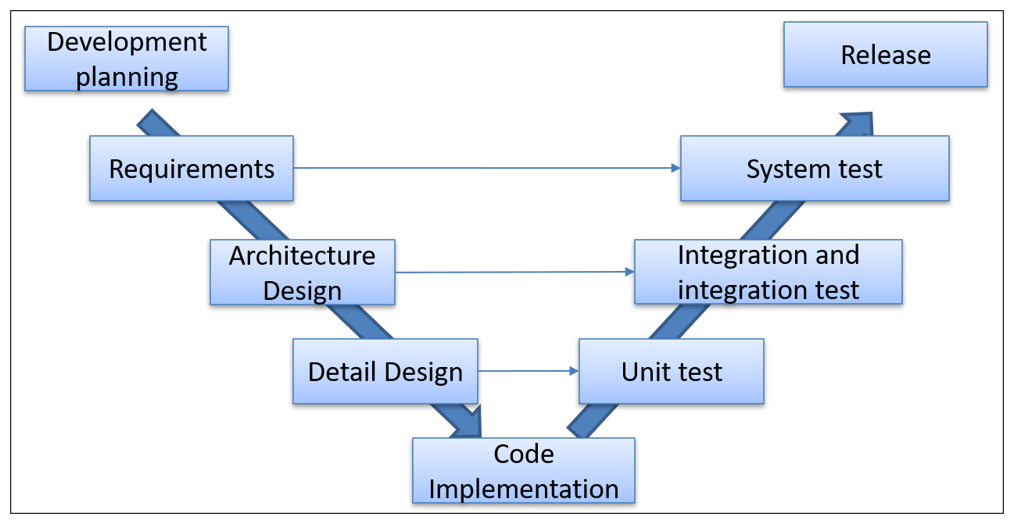
All You Need To Know About IEC 62304 Development For 41% OFF

Navigating IEC 62304 Standard for Medical Software CLEIO
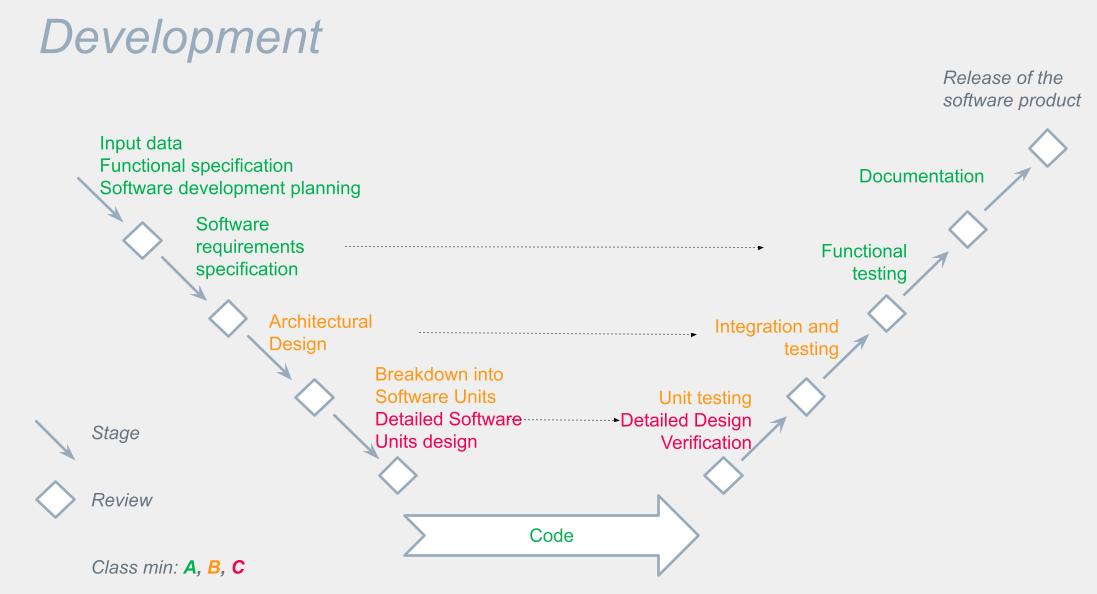
IEC 62304 Medical device Software life cycle

IEC 62304 Medical device Software life cycle

How to Apply IEC 62304 Requirements for Medical Device Software

IEC 82304 vs IEC 62304: Software Standards for SaMD Explained
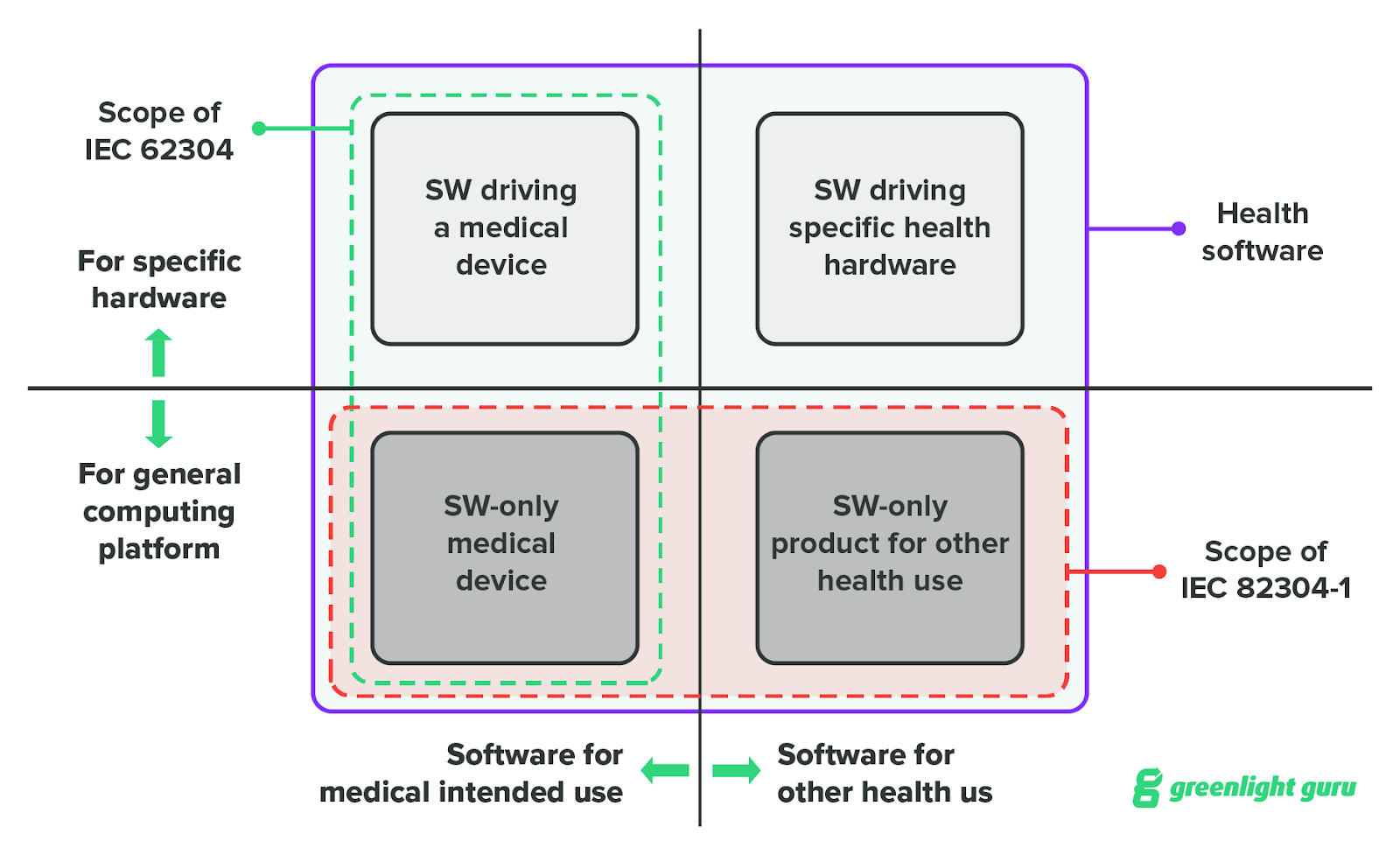
IEC 82304 vs IEC 62304: Software Standards for SaMD Explained

IEC 62304 How to Overcome Challenges in the Medtech?
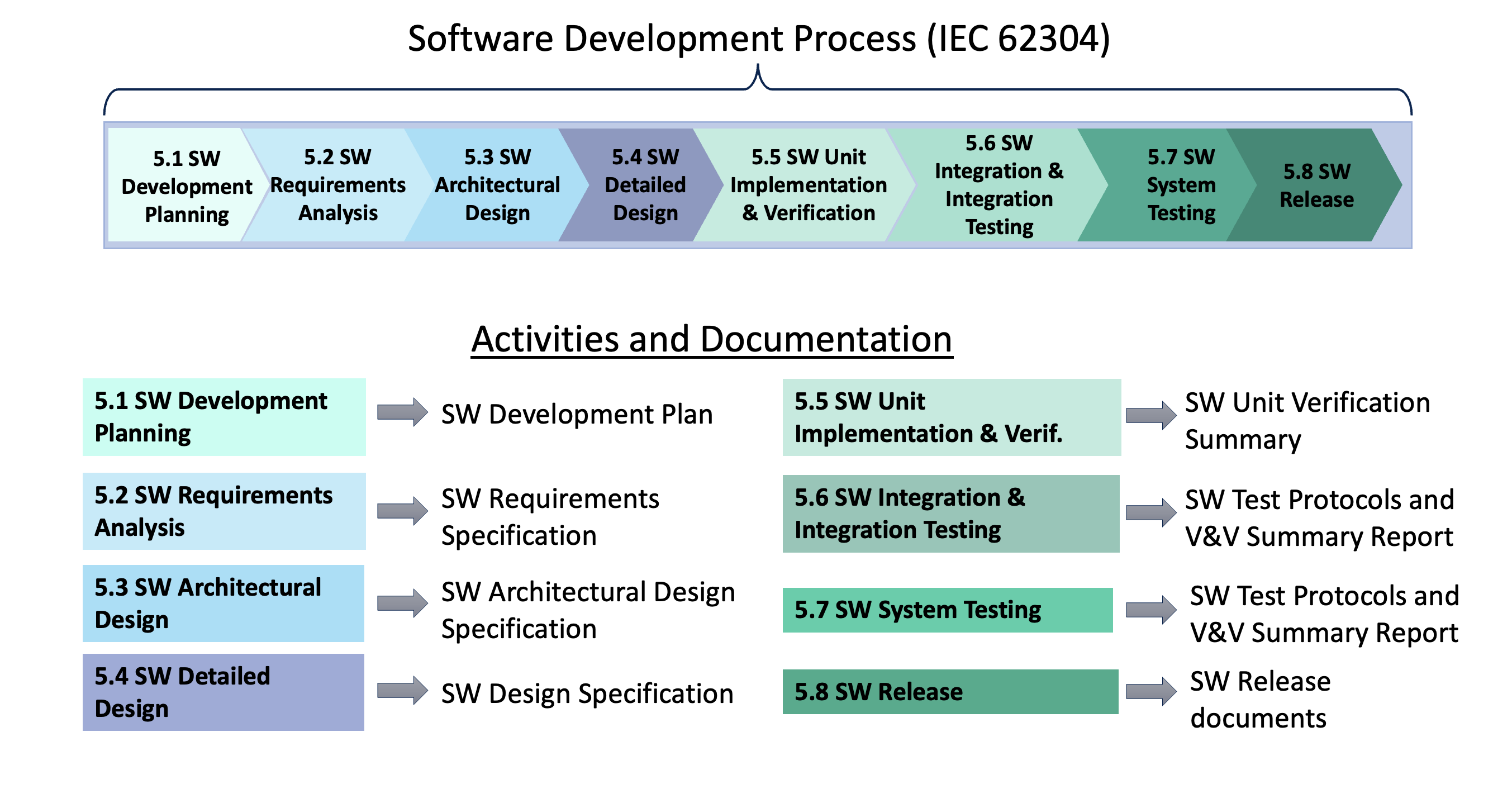
IEC62304 SW Dev Process and Docs Sunstone Pilot Inc
FDA Software Guidances and the IEC 62304 Software Standard Sunstone

The IEC 62304 standard and configuration management Medical Device HQ

Plantilla IEC 62304 Software Development Plan de Marko Drndarevic
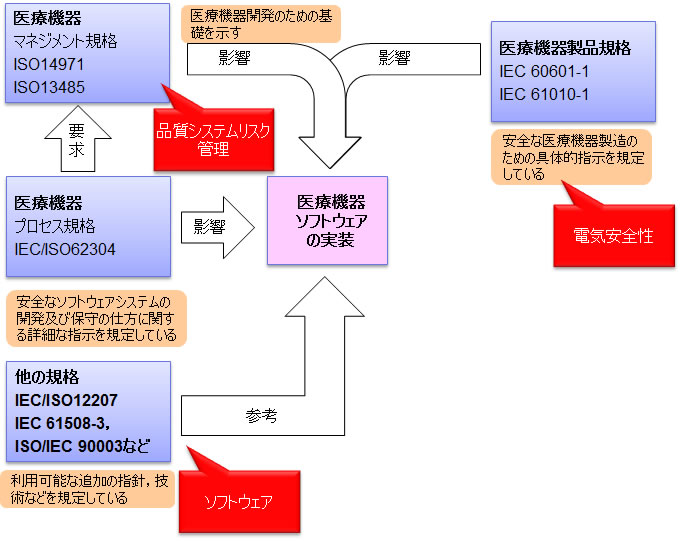
62304 Iso

62304 Iso

62304 Iso

62304 Iso

Introduction to SaMD IEC 62304 and IEC 82304 1 online course

An In Depth Guide to IEC 62304 Jama Software

Software Development Plan According to IEC 62304

Software Development Plan According to IEC 62304

IEC 62304 Software Norm für Medizinprodukte: Übersicht inovex GmbH

Software Safety Classification Template (IEC 62304 Medical Device

Software Safety Classification Template (IEC 62304 Medical Device

Software Safety Classification Template (IEC 62304 Medical Device
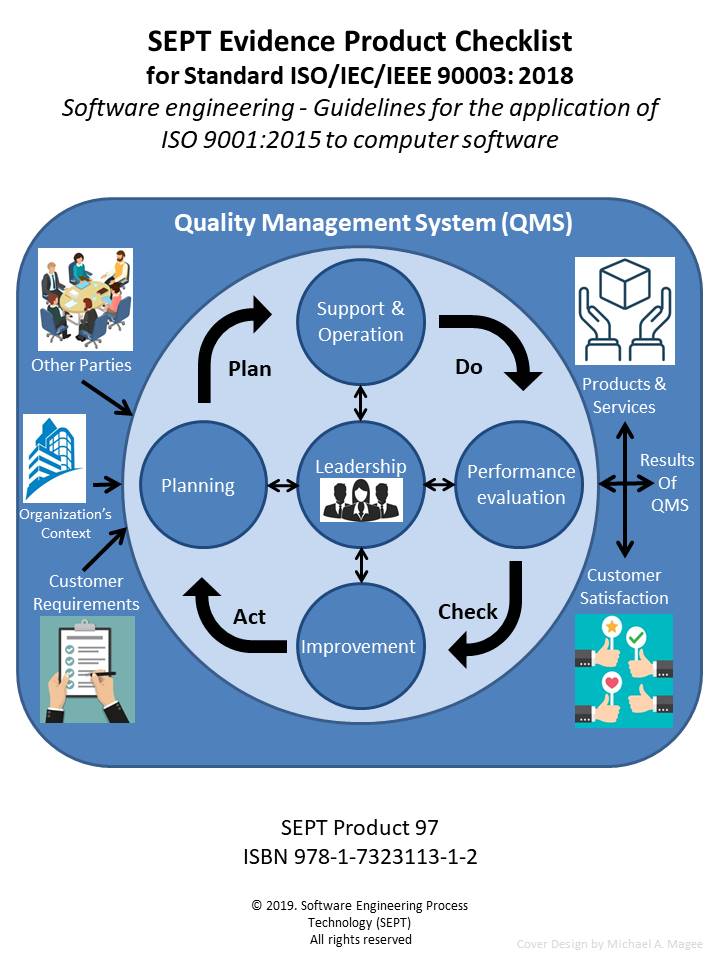
Software Maintenance Plan Template Life Cycle Processes ISO/IEC 14764
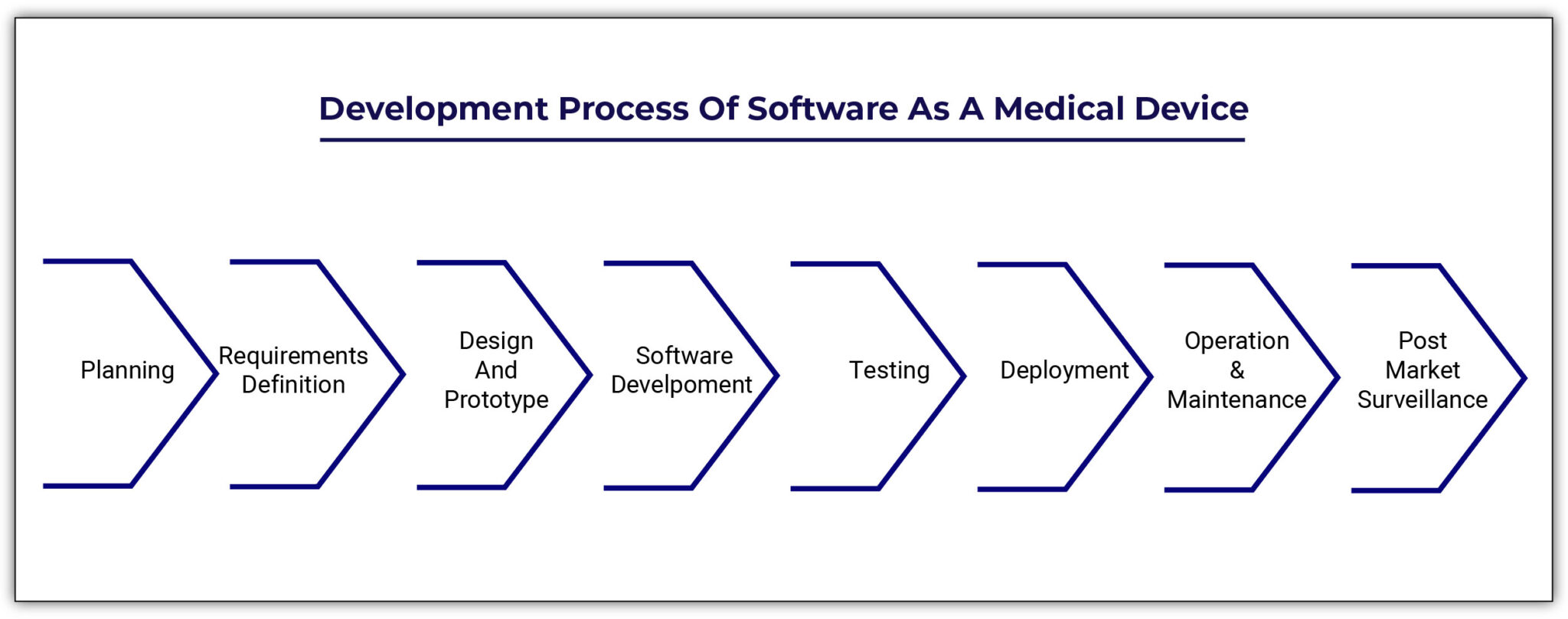
Software as a Medical Device (SaMD) IEC 62304 Certification

Implementing IEC 62304 for Safe and Effective Medical Device Software
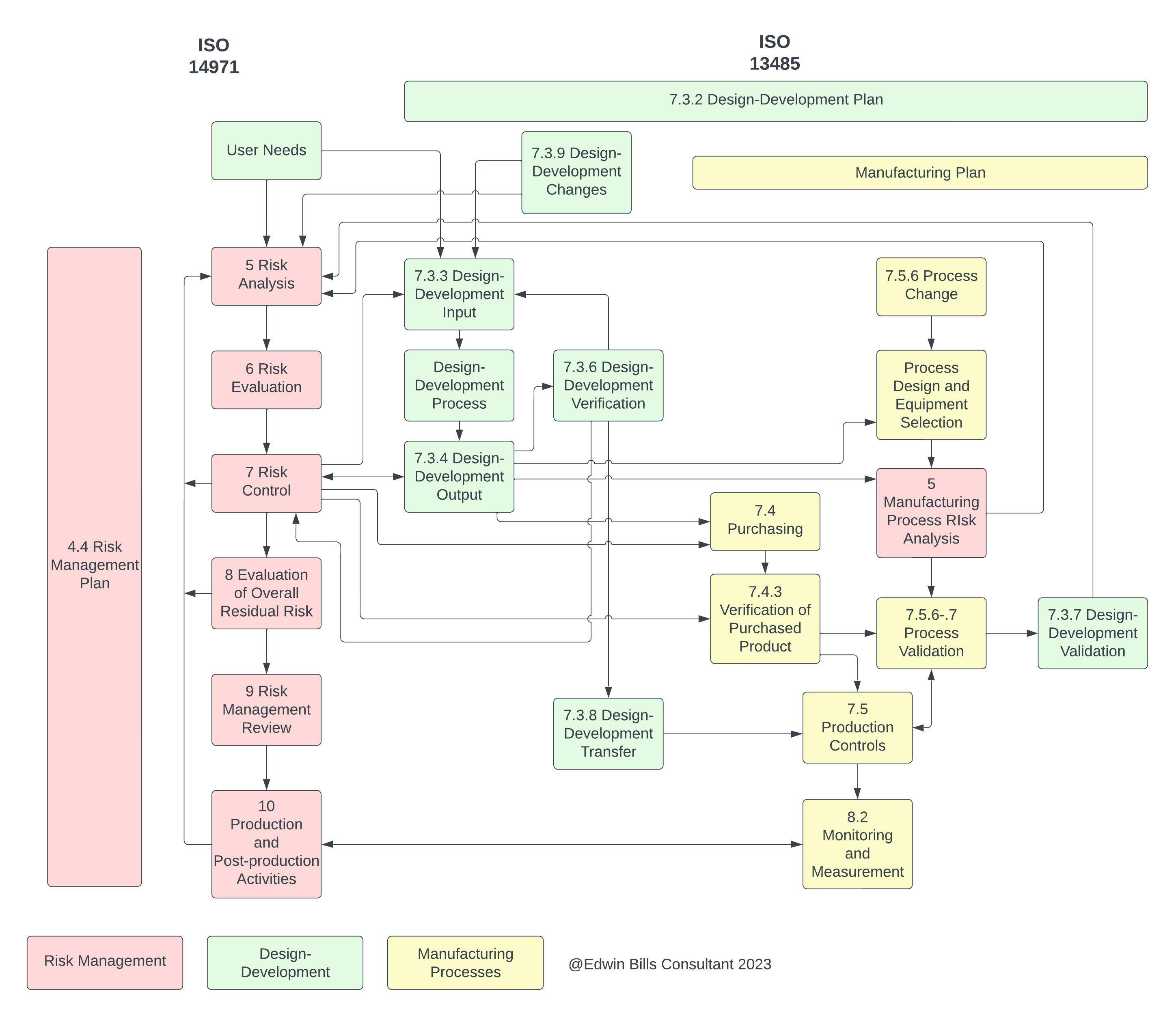
Fda 13485 Requirements

IEC 62304 CheckList word文档在线阅读与下载 免费文档
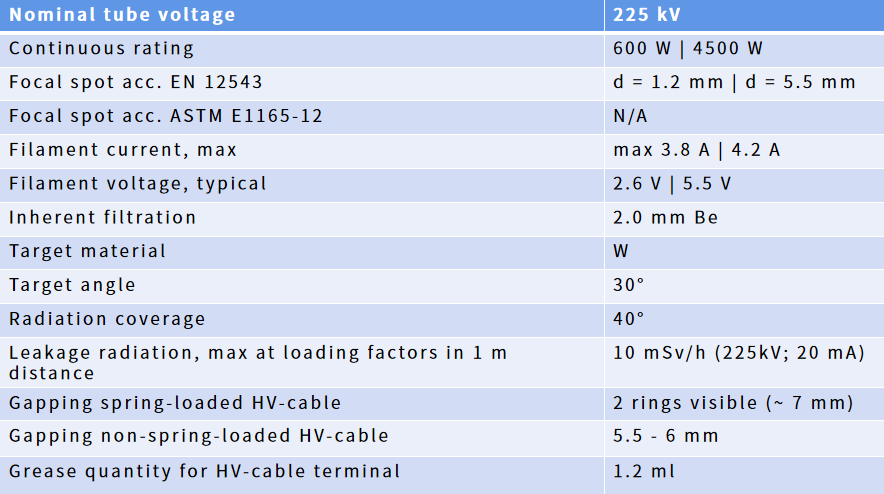
SEMISHARE Introduction of the Integrated Solution for X ray Irradiation