Regulatory Strategy Template For Medical Devices
Here are some of the images for Regulatory Strategy Template For Medical Devices that we found in our website database.

Regulatory Strategy Template For Medical Devices

Regulatory Strategy Template For Medical Devices

Regulatory Strategy Template For Medical Devices

Regulatory Strategy Template For Medical Devices
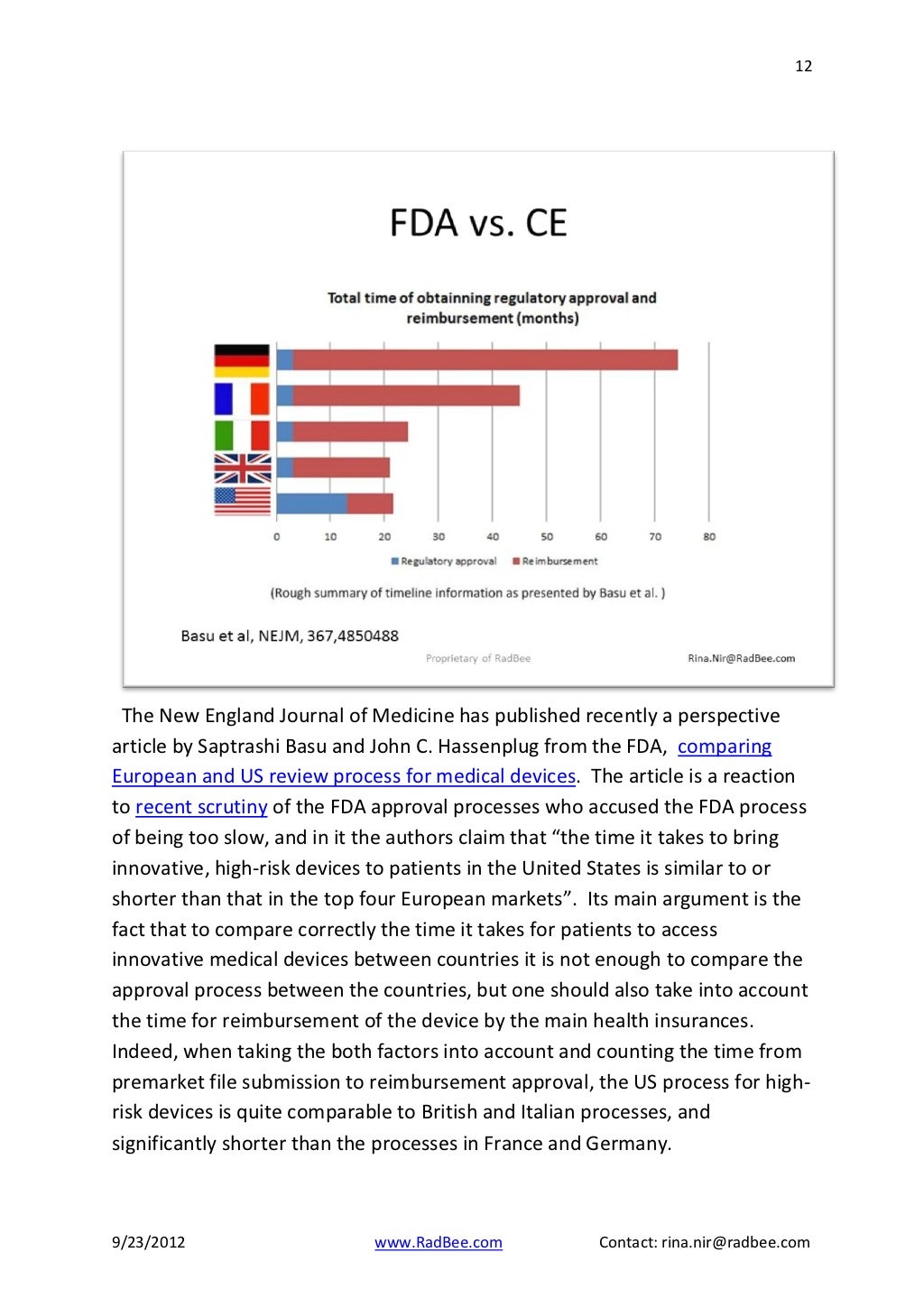
Regulatory Strategy Template For Medical Devices

Regulatory Strategy Template For Medical Devices

Regulatory Strategy Template For Medical Devices
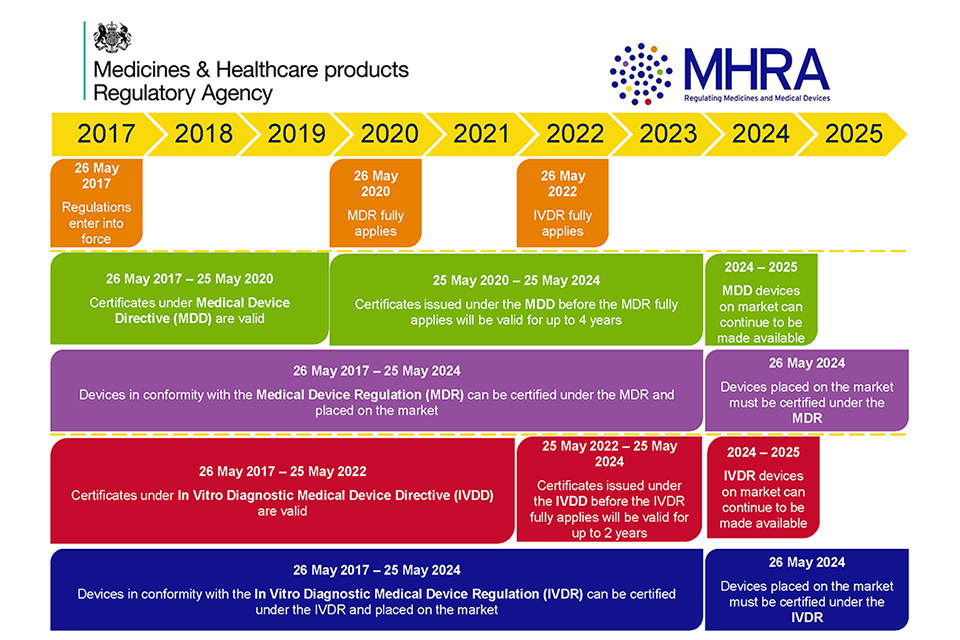
Regulatory Strategy Template For Medical Devices
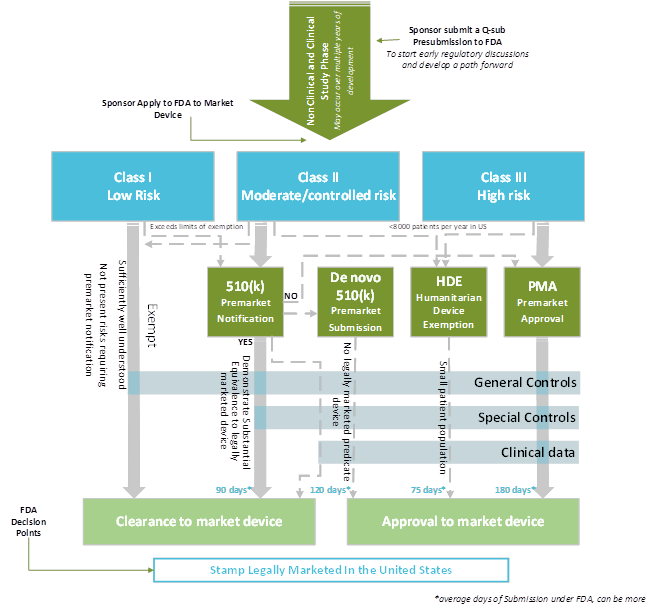
Regulatory Strategy Template For Medical Devices
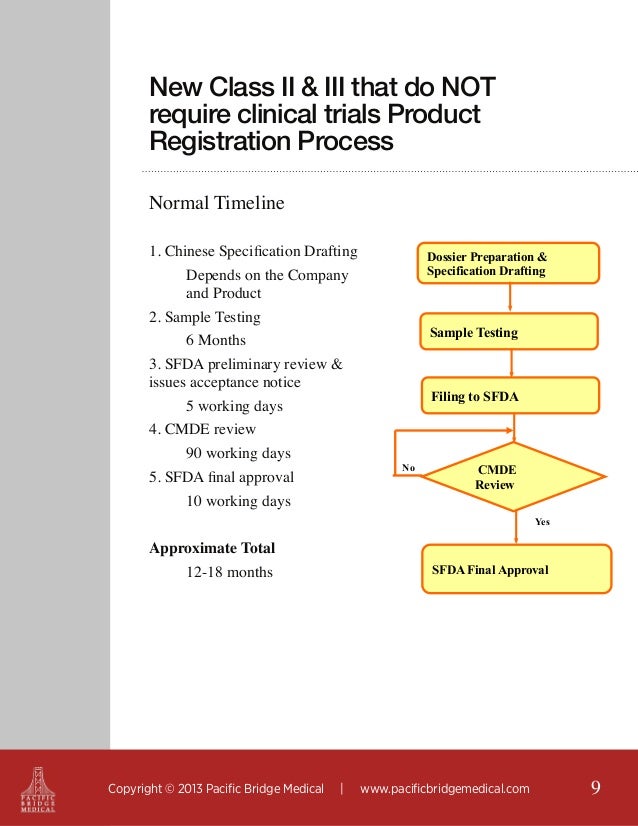
Regulatory Strategy Template For Medical Devices

Webinar: Regulatory Strategy for New Neurological Medical Devices
Cofepris New Regulatory Safety Strategy for Medical Devices

Regulatory Compliance Strategy for Medical Devices Corpbiz

The Complete Regulatory Pathway for Medical Devices:
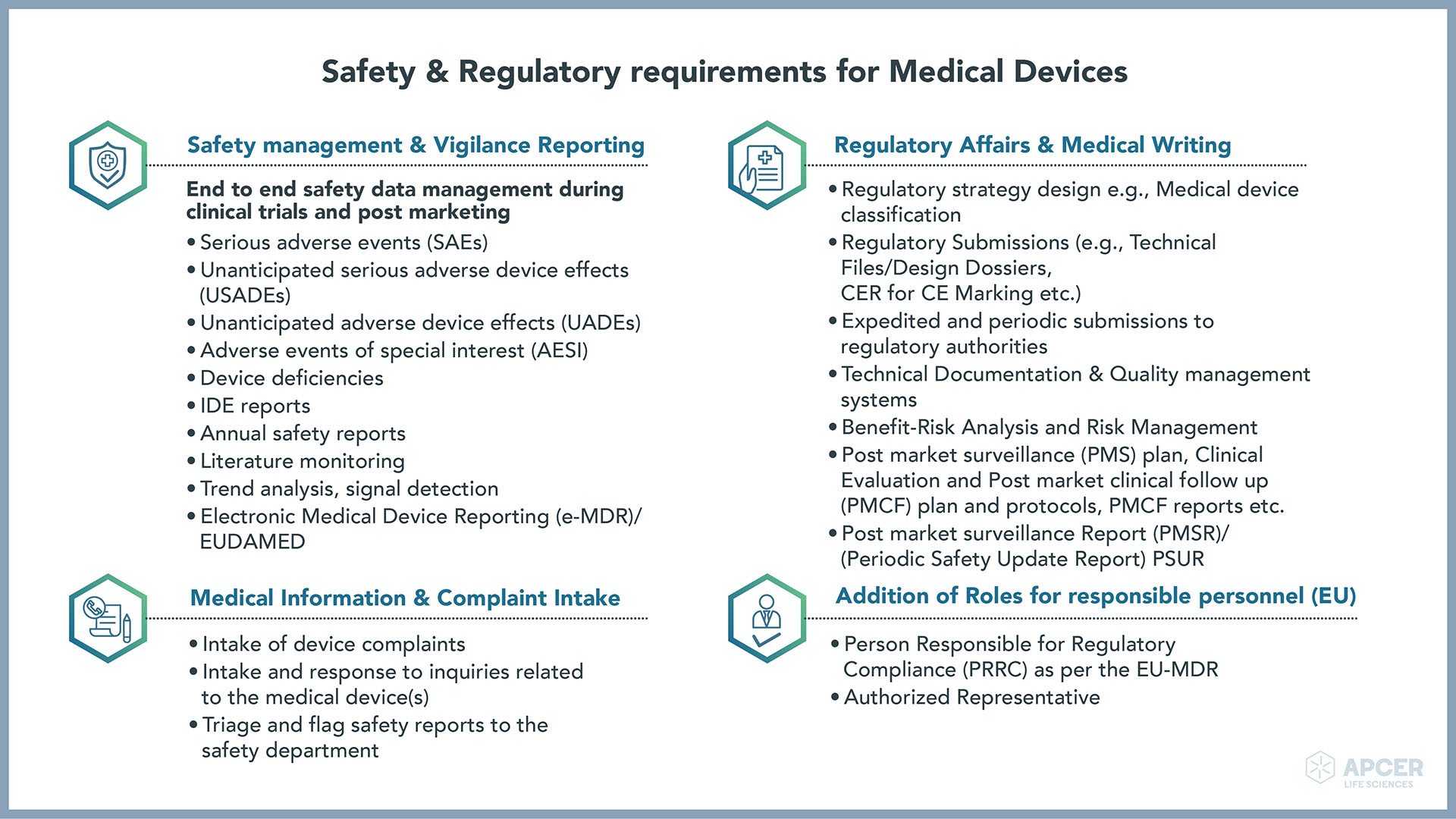
Safety Regulatory requirements for Medical Devices APCER Life Sciences
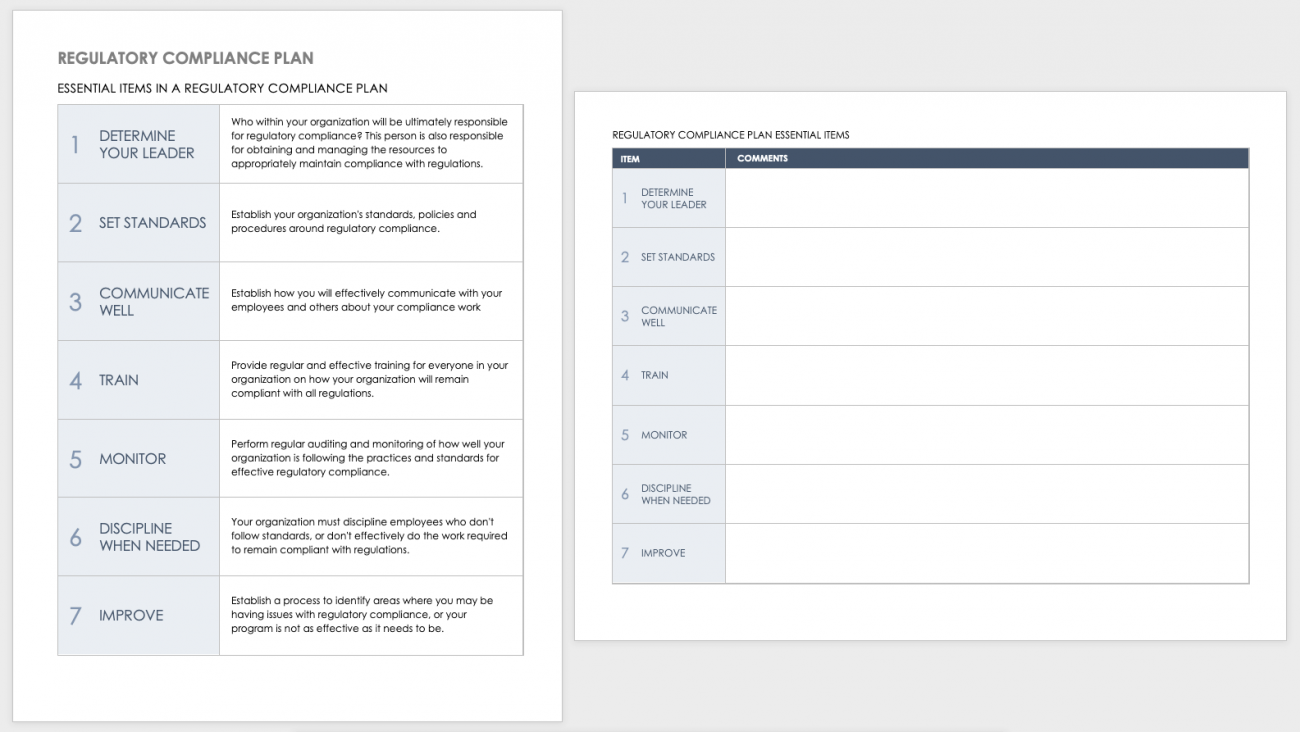
Regulatory Strategy Template

Regulatory Strategy Template
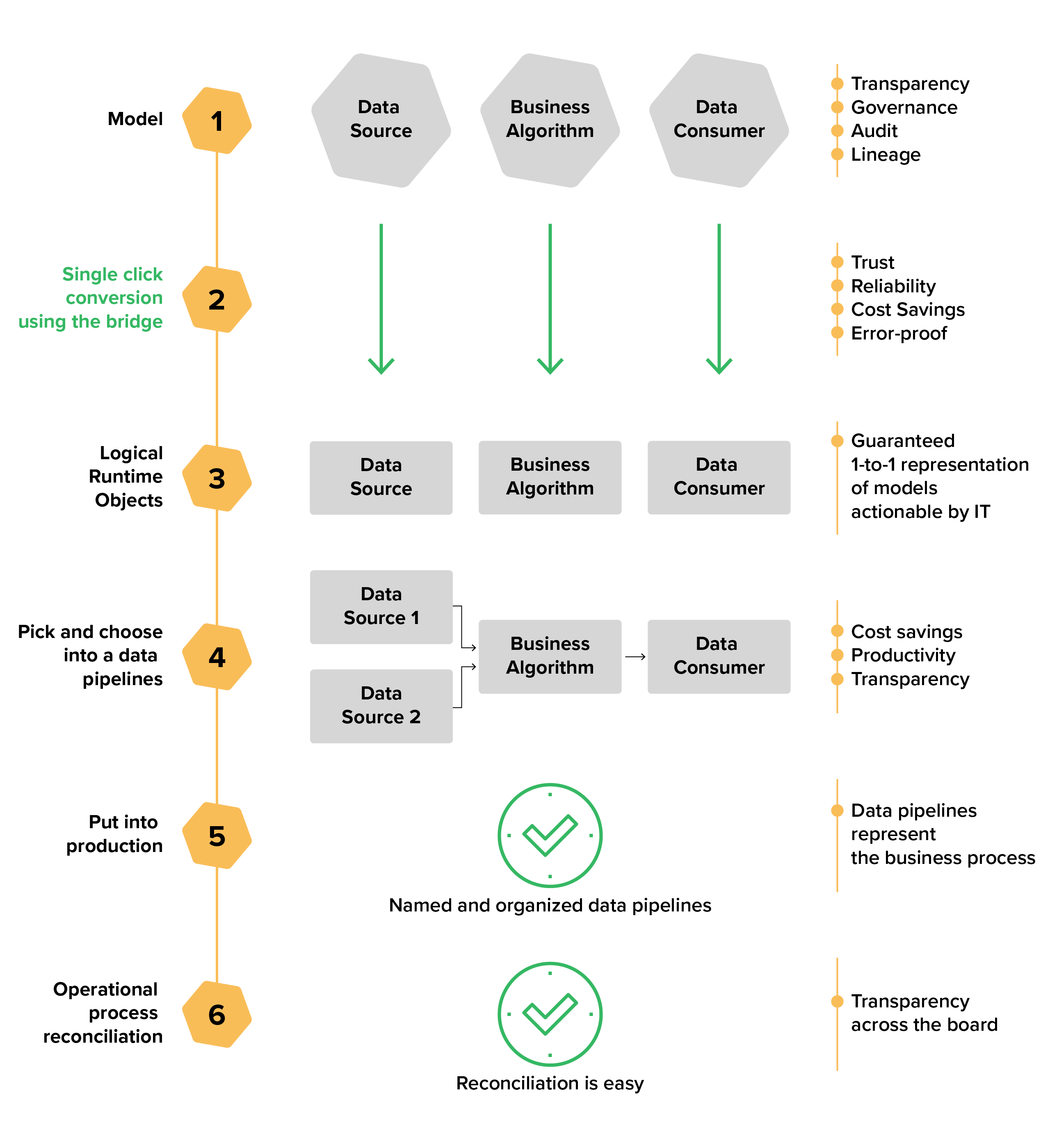
Regulatory Strategy Template

Regulatory Strategy Template

Administration Regulatory Submission Strategy Plan Template Edit

In Depth Regulatory Affairs Strategy Advertising Document Template in

Regulatory Strategy Template

Regulatory Strategy Template
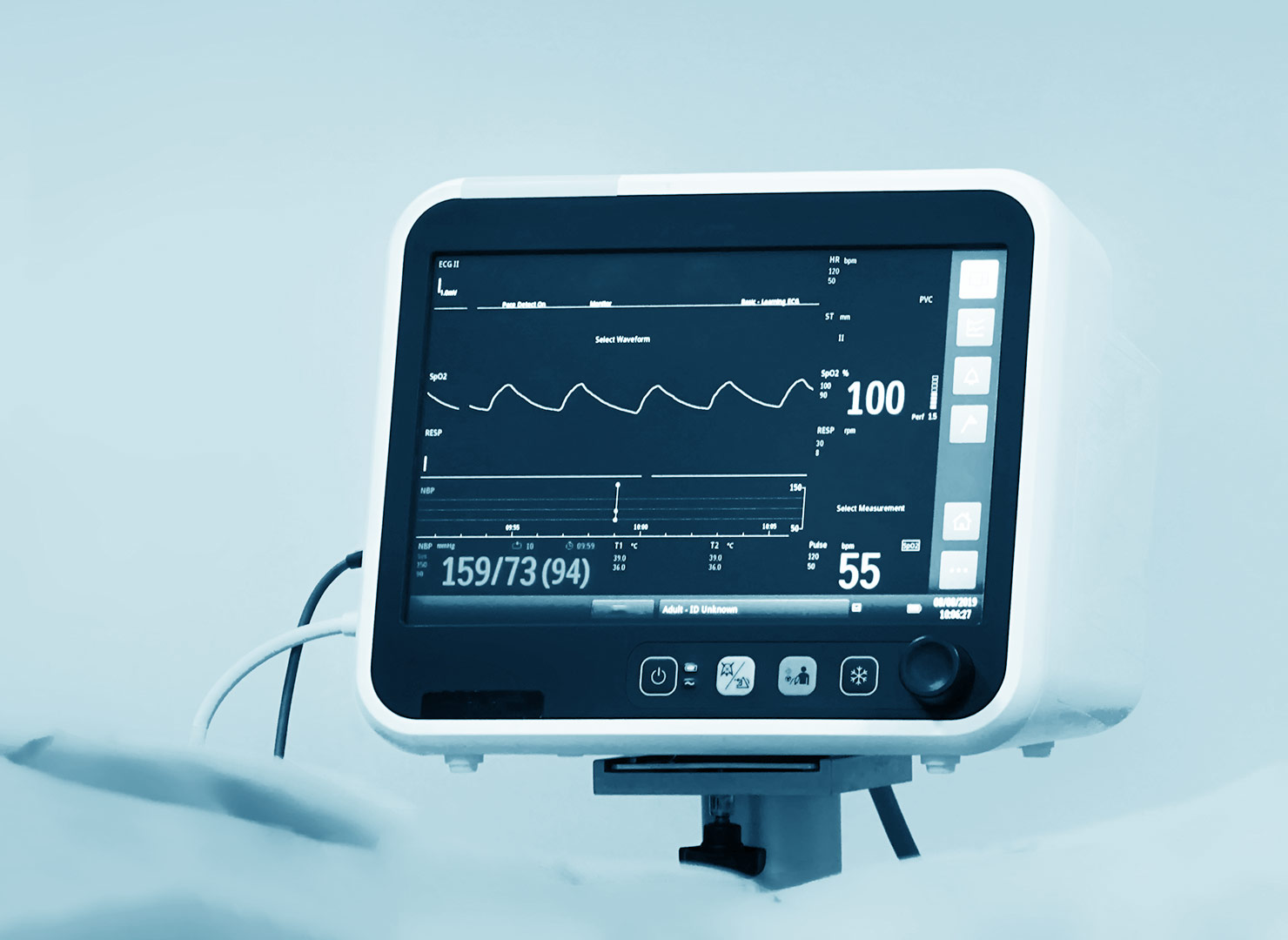
How to Develop a Robust Regulatory Strategy for Medical Devices Step

The 4 key principles of a smart regulatory strategy for medical devices

Regulatory Plan Template Printable Word Searches

Regulatory Requirements Database for Medical Devices DDi

Creating a Medical Device Regulatory Strategy: Key Components

Regulatory Plan Template prntbl concejomunicipaldechinu gov co

MDR Regulatory Strategy: implementation guide · MDlaw Information

Developing a regulatory strategy: 6 good reasons
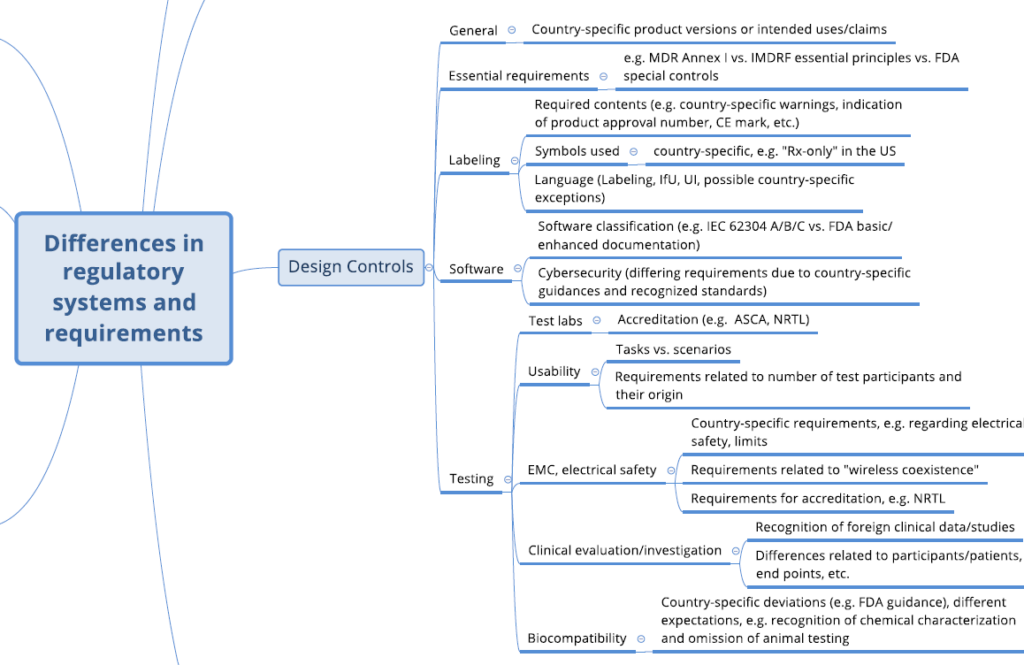
Developing a regulatory strategy: 6 good reasons
Regulatory Plan Template prntbl concejomunicipaldechinu gov co
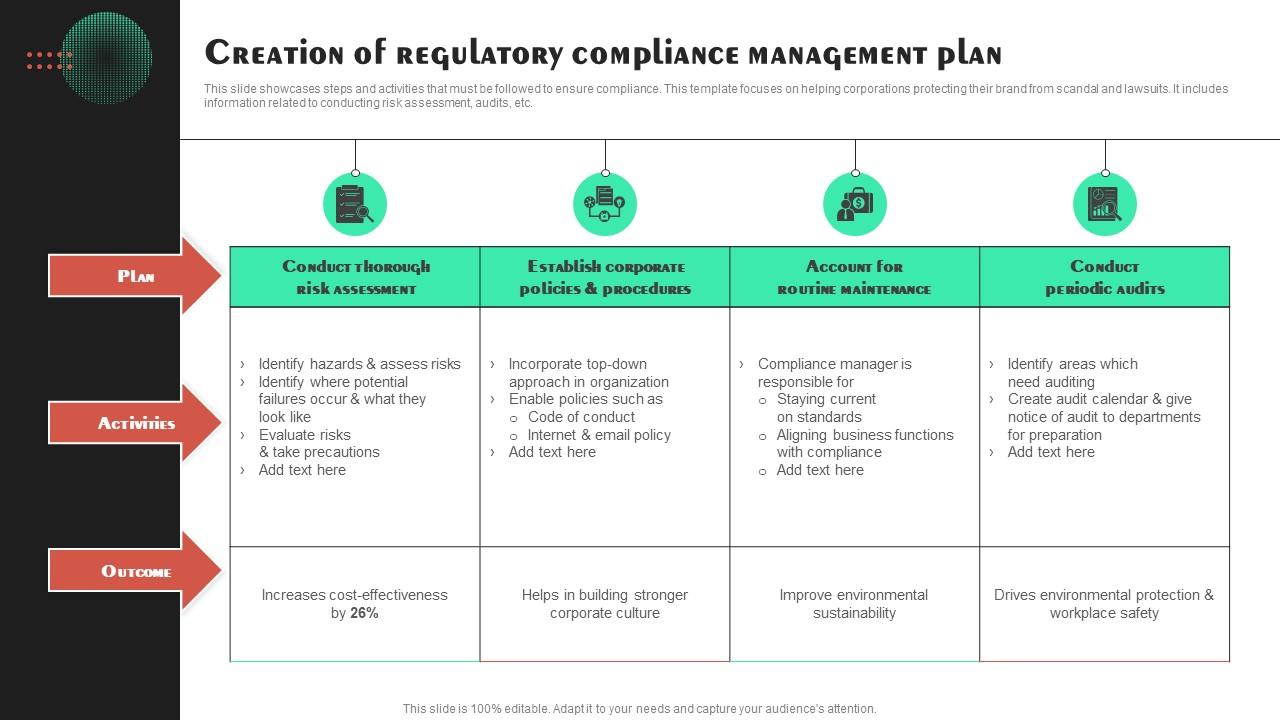
Regulatory Plan Template prntbl concejomunicipaldechinu gov co

Regulatory Plan Template prntbl concejomunicipaldechinu gov co

Regulatory Plan Template prntbl concejomunicipaldechinu gov co

Regulatory Plan Template prntbl concejomunicipaldechinu gov co

Regulatory Plan Template prntbl concejomunicipaldechinu gov co
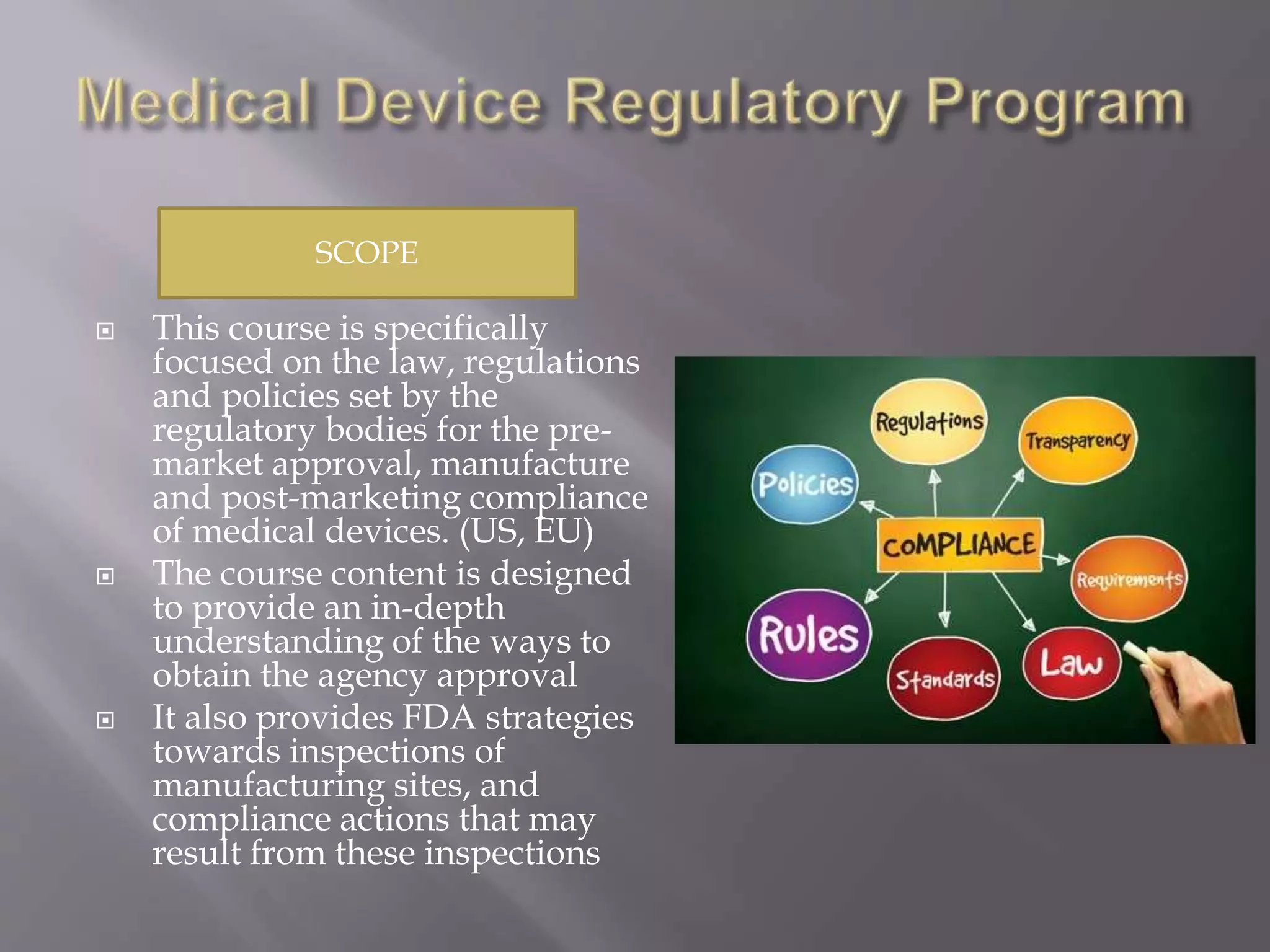
Medical Device Regulatory Affairs PPTX

ISO 13485 QMS Template Packages Launch LFH Regulatory
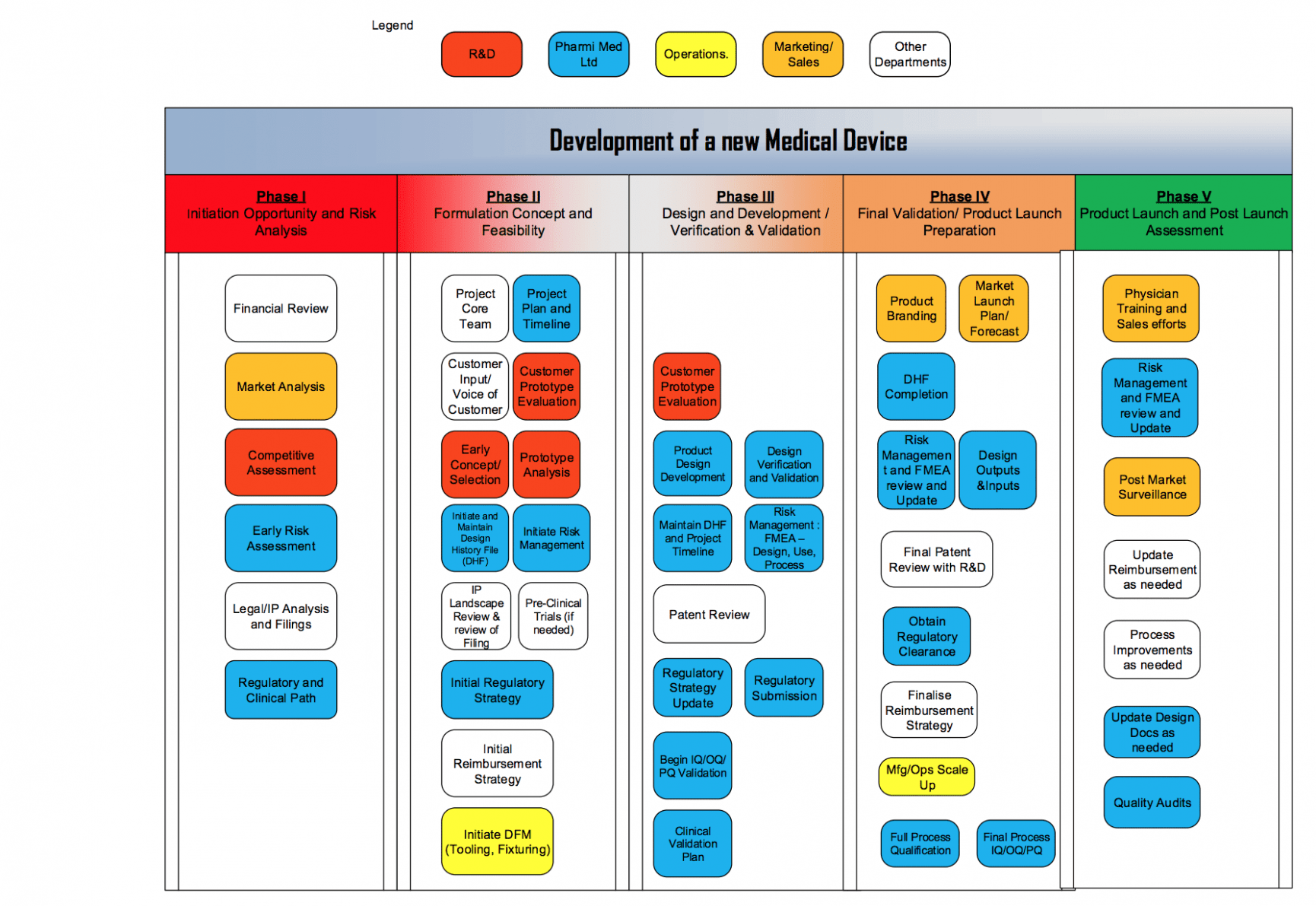
Medical Device Design And Development Plan Template

Five Years Medical Device Regulatory Product Approval Roadmap

Medical Device Regulatory Services UKCA CE and FDA Hardian Health

Regulatory Strategy for Medical Devices: Global Market Planning
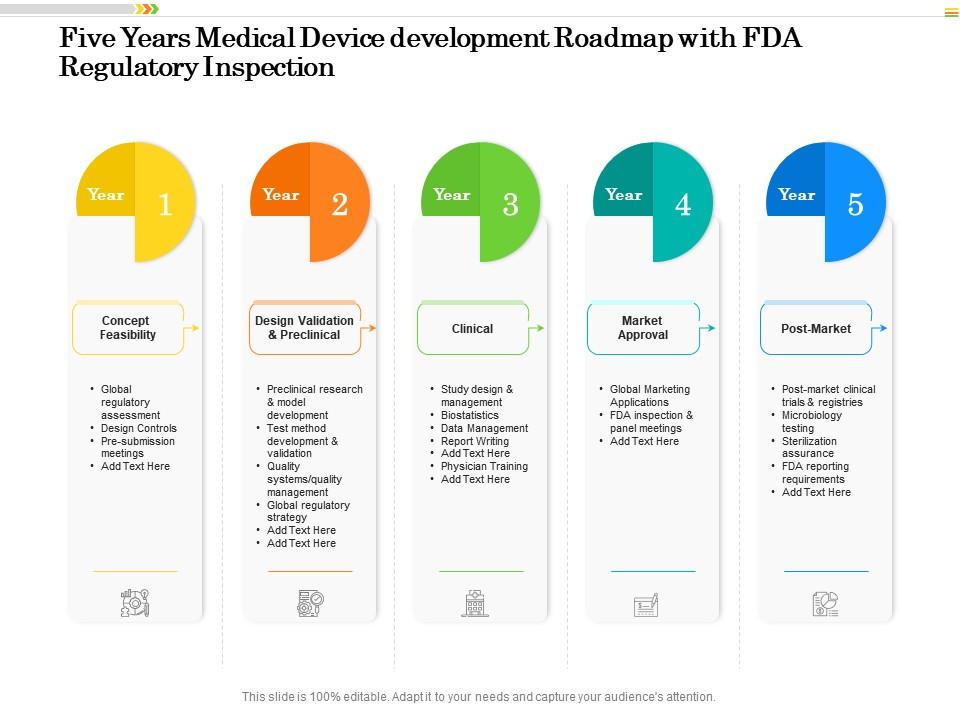
Five Years Medical Device Development Roadmap With FDA Regulatory
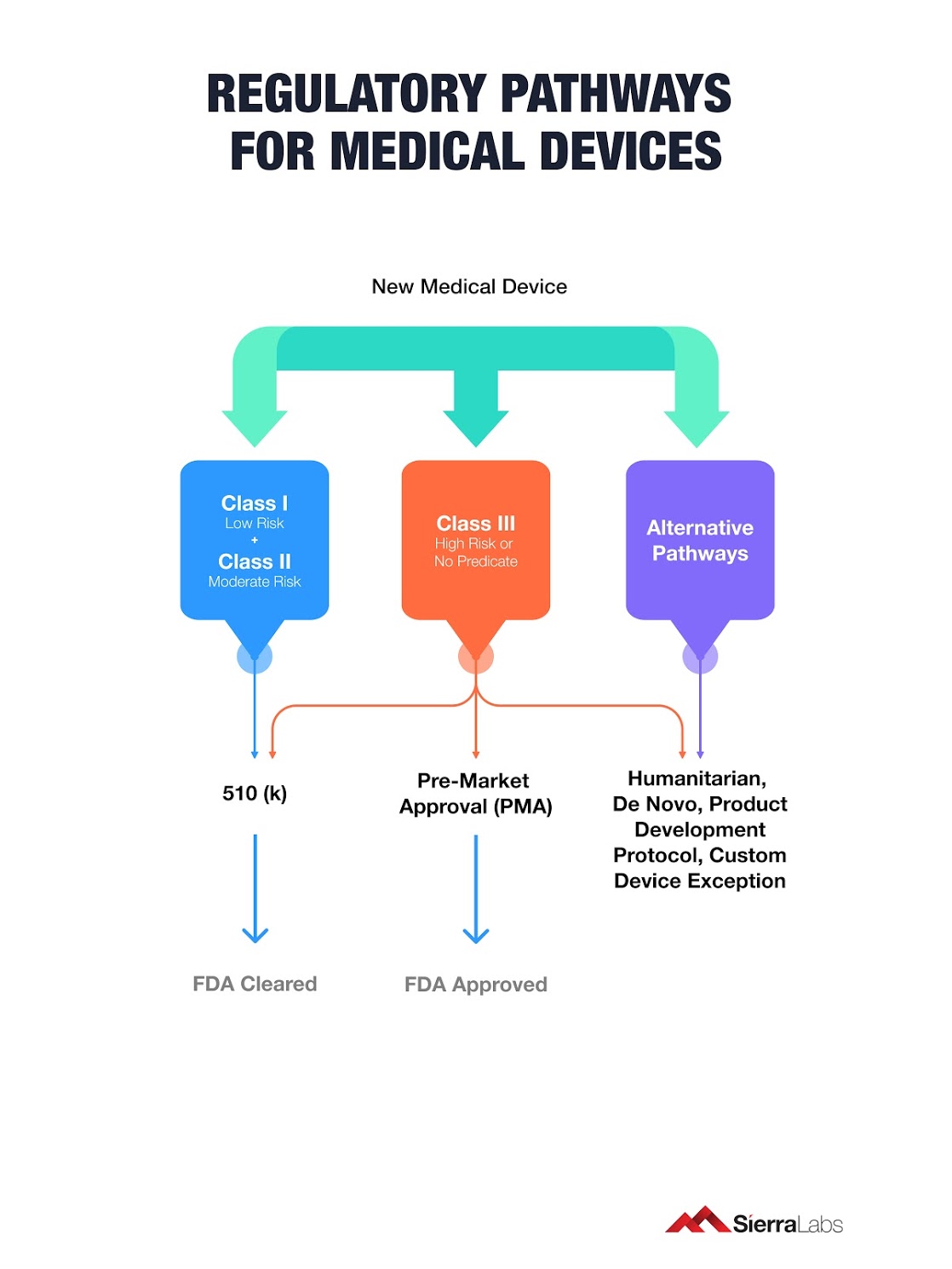
6 Regulatory Pathways to Bring Your Medical Device to Market
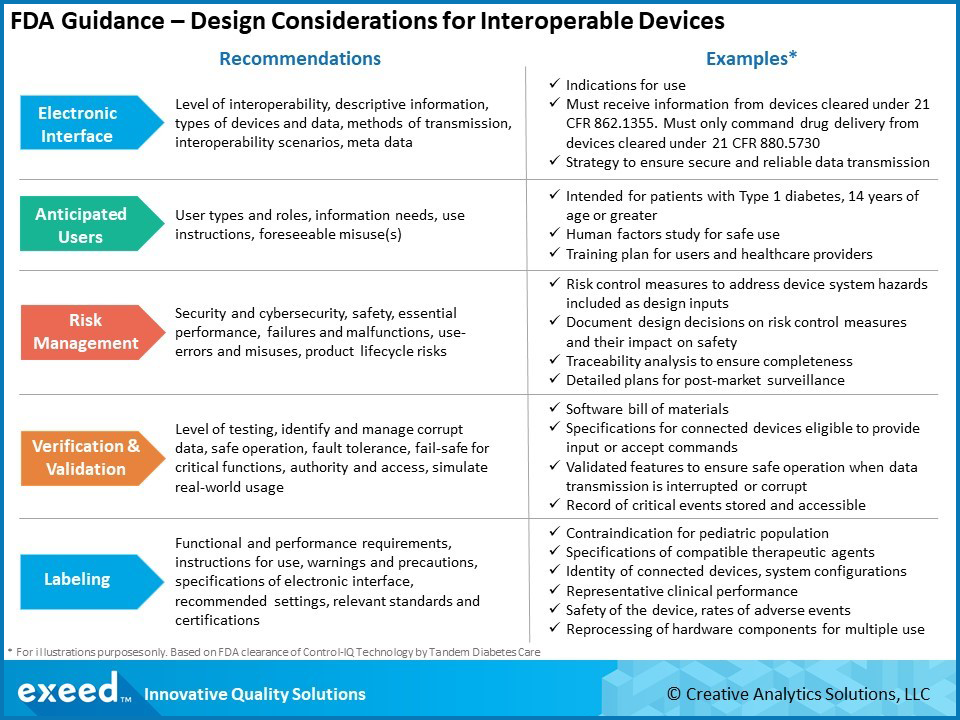
FDA Guidance Shows a Regulatory Path Forward for Interoperable Devices

Why is a regulatory strategy important for medical devices?

Regulatory Strategy For Drug Development Ppt Powerpoint Presentation

Six Months Roadmap Of Medical Device Approval From FDA Regulatory

EU Declaration of Conformity

Regulatory Affairs Specialist Cover Letter Examples QwikResume
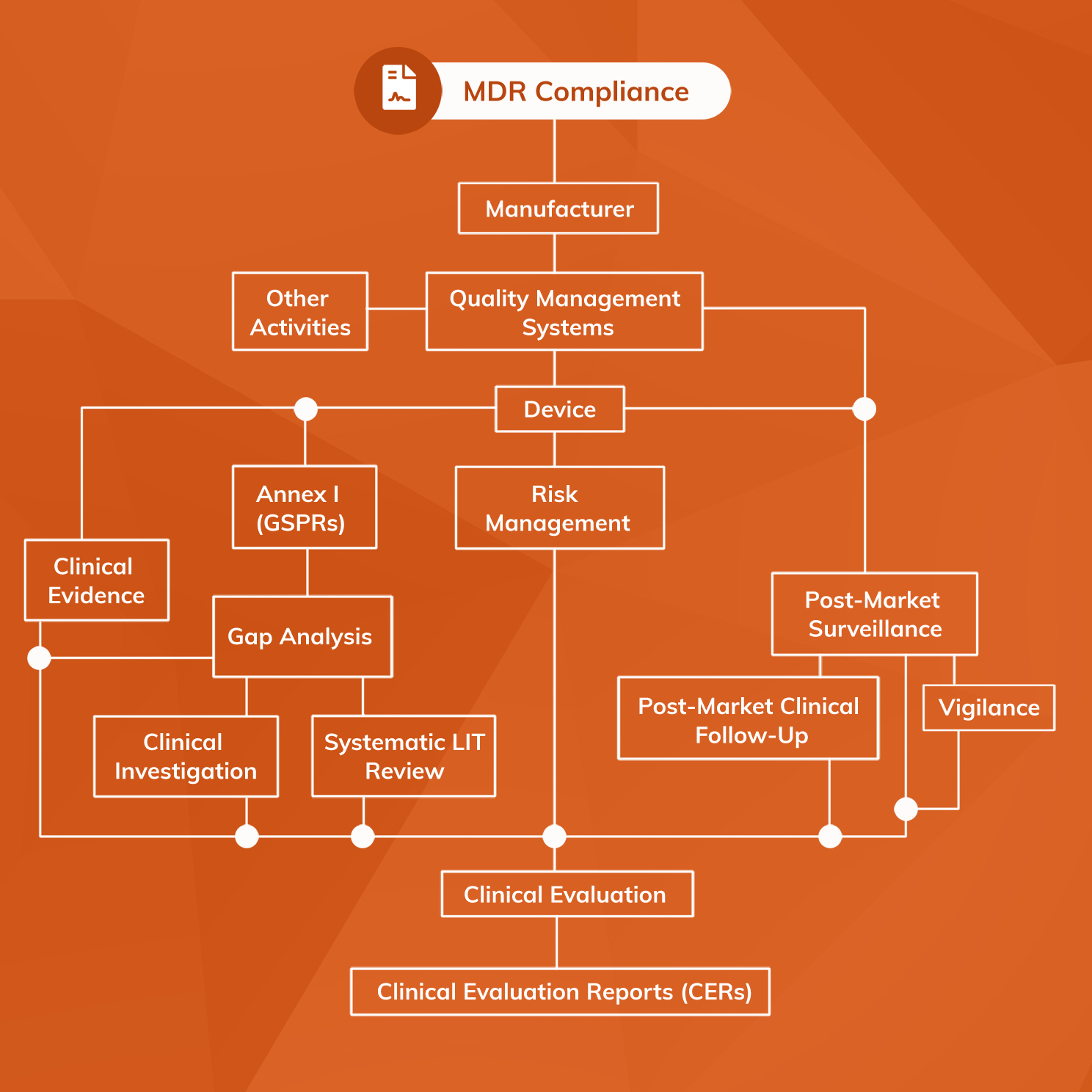
Software as a Medical Device (SaMD) MDR Compliance Guide Mantra Systems

Dec 2024: UK Publishes New Regulatory Roadmap Casus Consulting
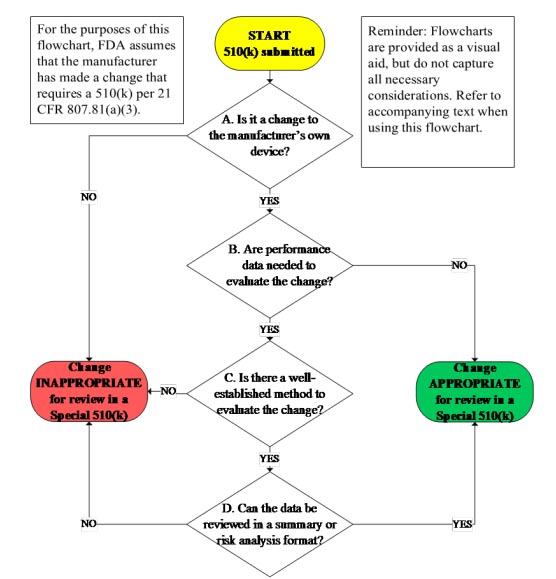
FDA Updates Several 510(k) Guidance Documents

16 Business Continuity Plan Templates For Every Business

The Regulation of Wearable Medical Devices: Trends in Biotechnology