Post Market Surveillance Plan Template
Here are some of the images for Post Market Surveillance Plan Template that we found in our website database.

Post Market Surveillance Plan PDF Surveillance Performance Indicator

PMS Plan Download a Free PMS Plan Template

PMS Plan Download a Free PMS Plan Template

Post Market Surveillance Plan Template Content Calendar Template

D 751 11 Post Market Surveillance Plan Report Template Vee Care Asia

Post Market Surveillance Plan Template prntbl concejomunicipaldechinu

Post Market Surveillance Plan Template
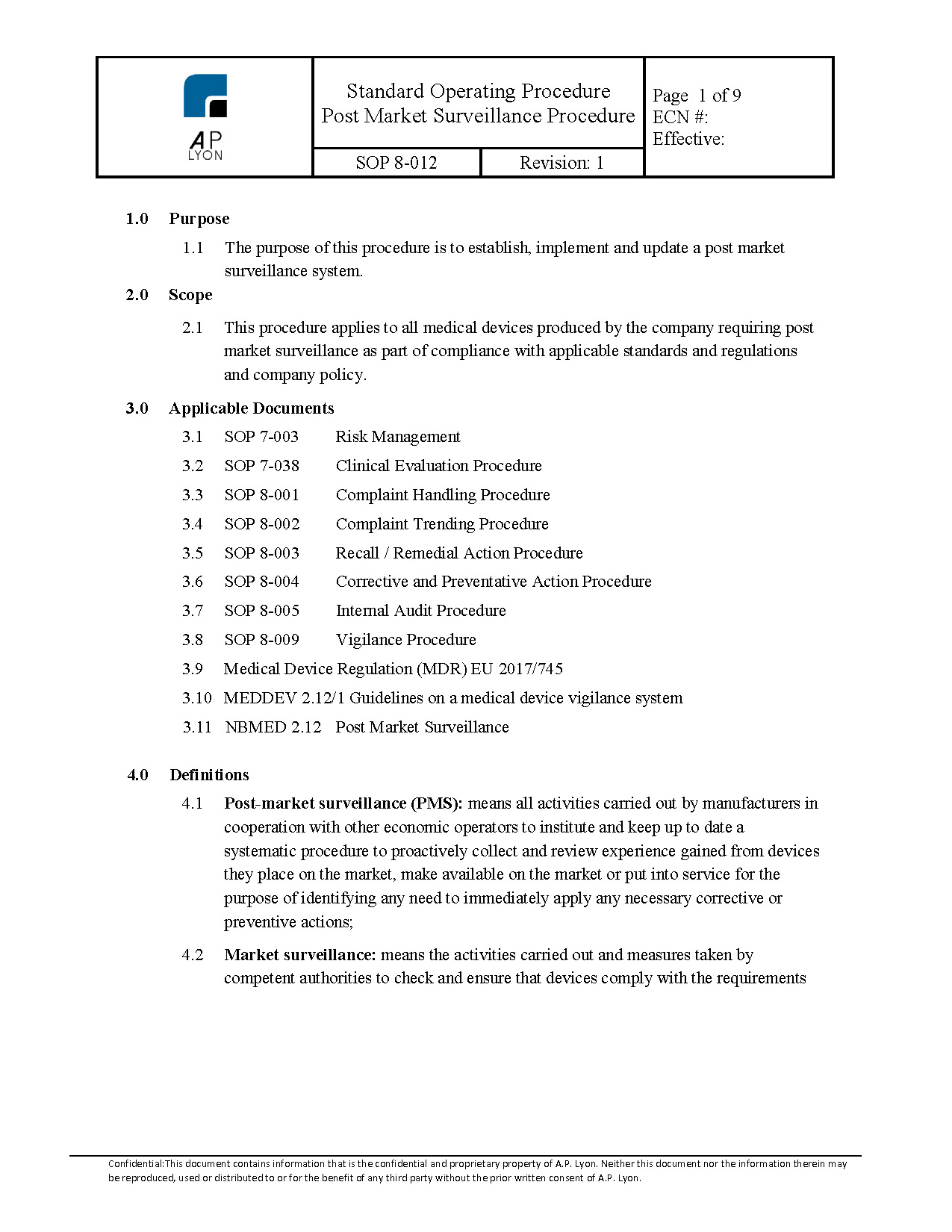
Post Market Surveillance Plan Template
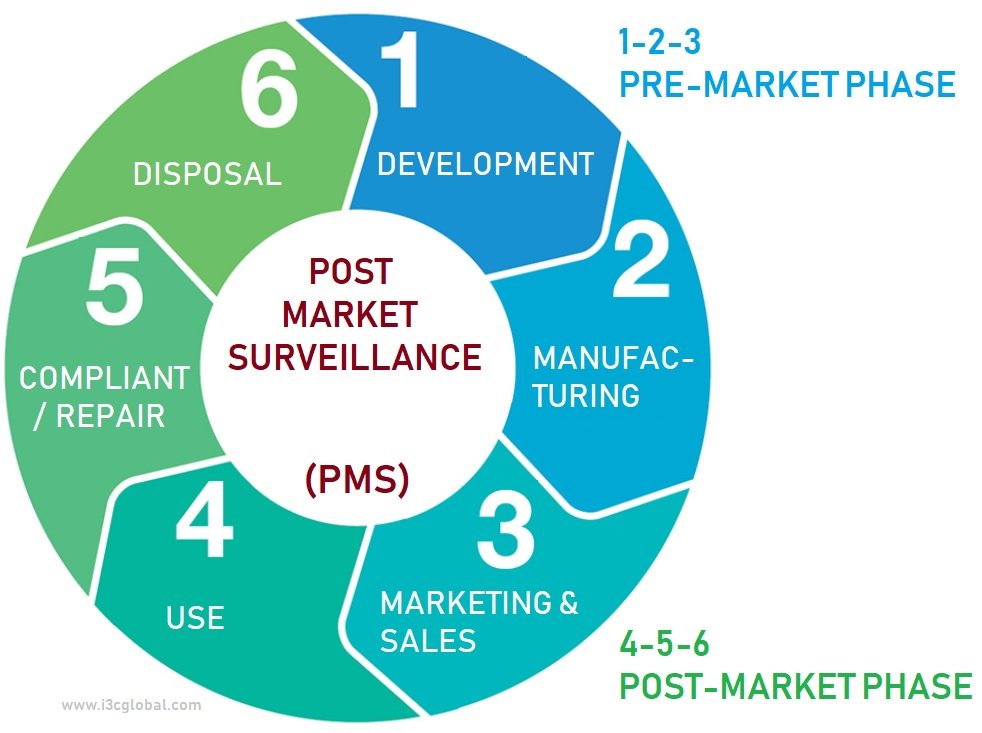
Post Market Surveillance Plan Template

Post Market Surveillance Plan Template
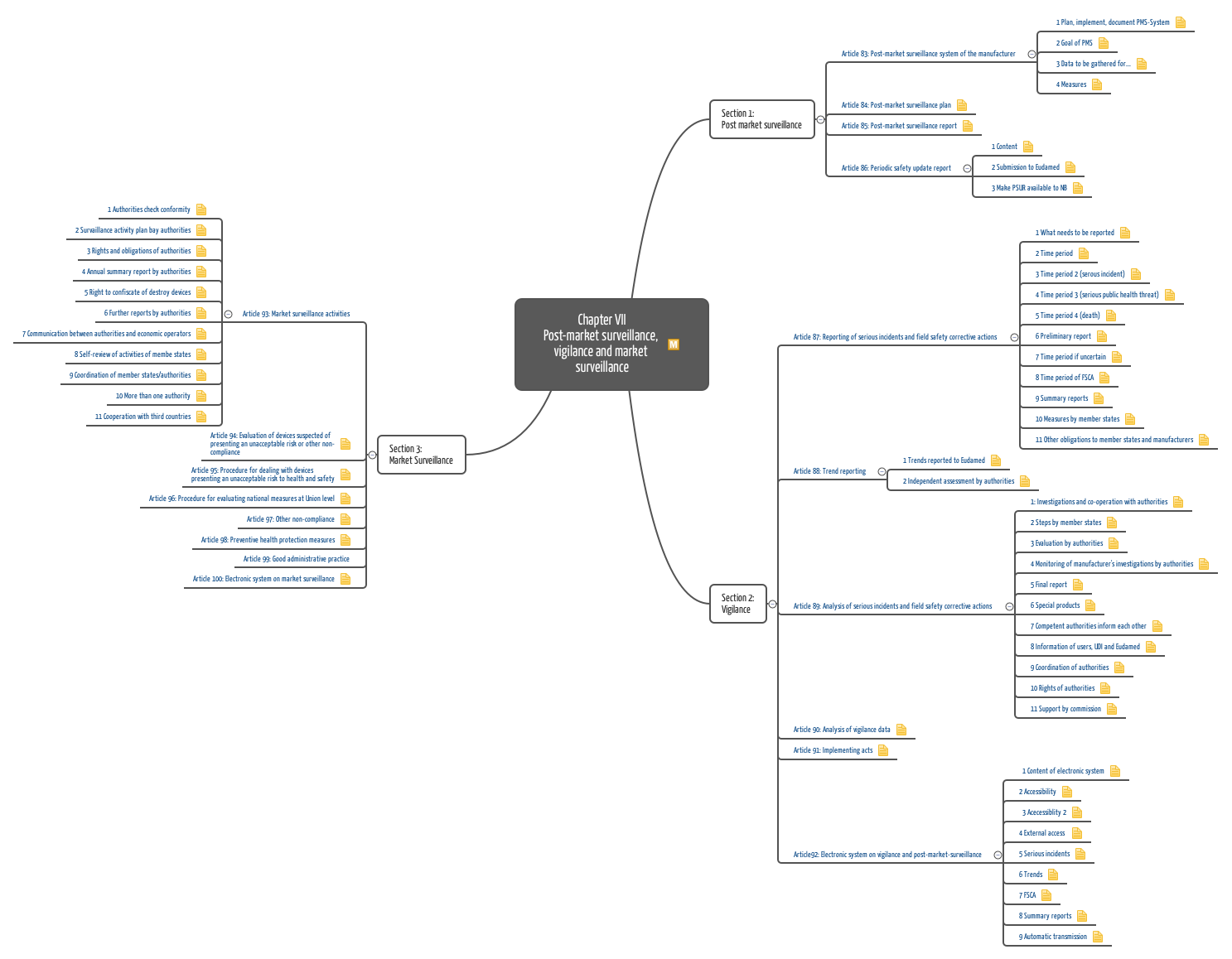
Post Market Surveillance Plan Template
Post Market Surveillance Plan Template
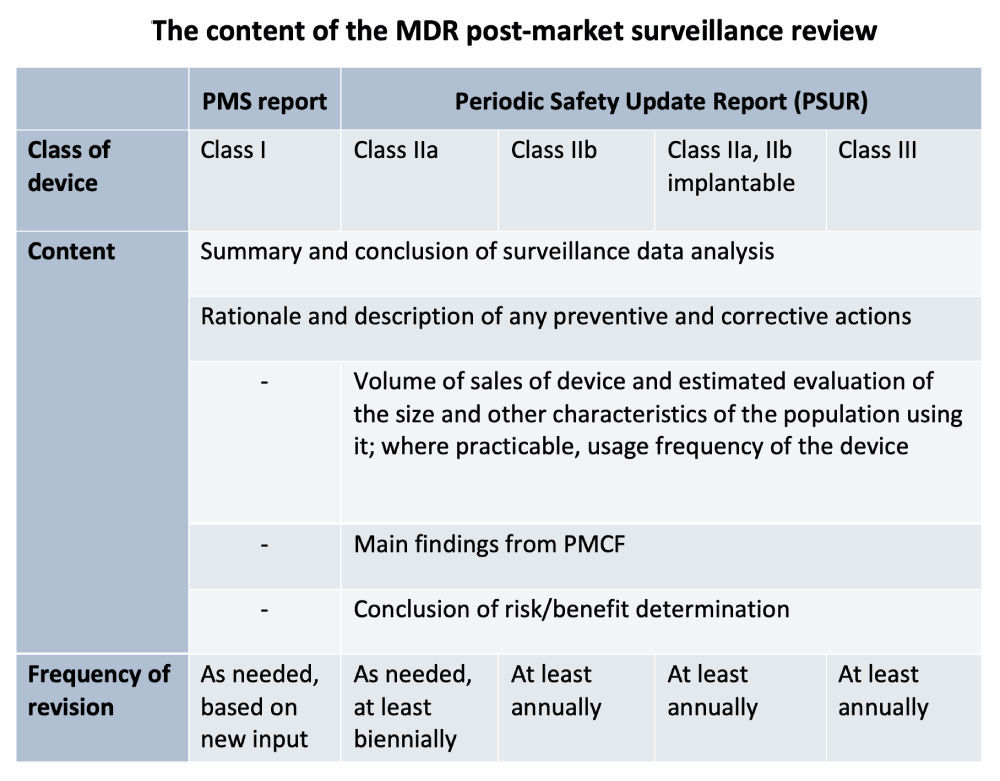
Post Market Surveillance Plan Template prntbl concejomunicipaldechinu
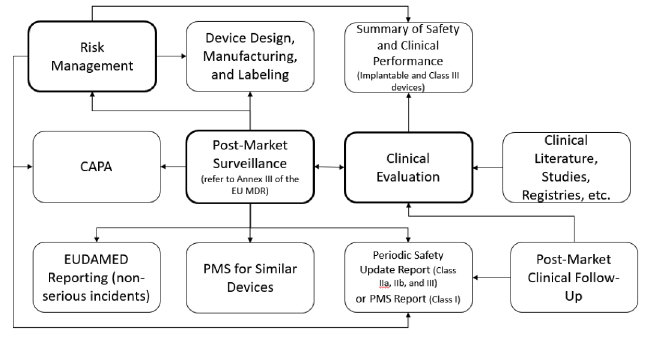
Post Market Surveillance Plan Template prntbl concejomunicipaldechinu
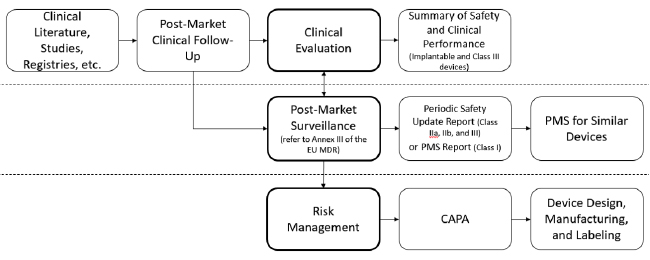
Post Market Surveillance Plan Template prntbl concejomunicipaldechinu

Post Market Surveillance Plan Template prntbl concejomunicipaldechinu
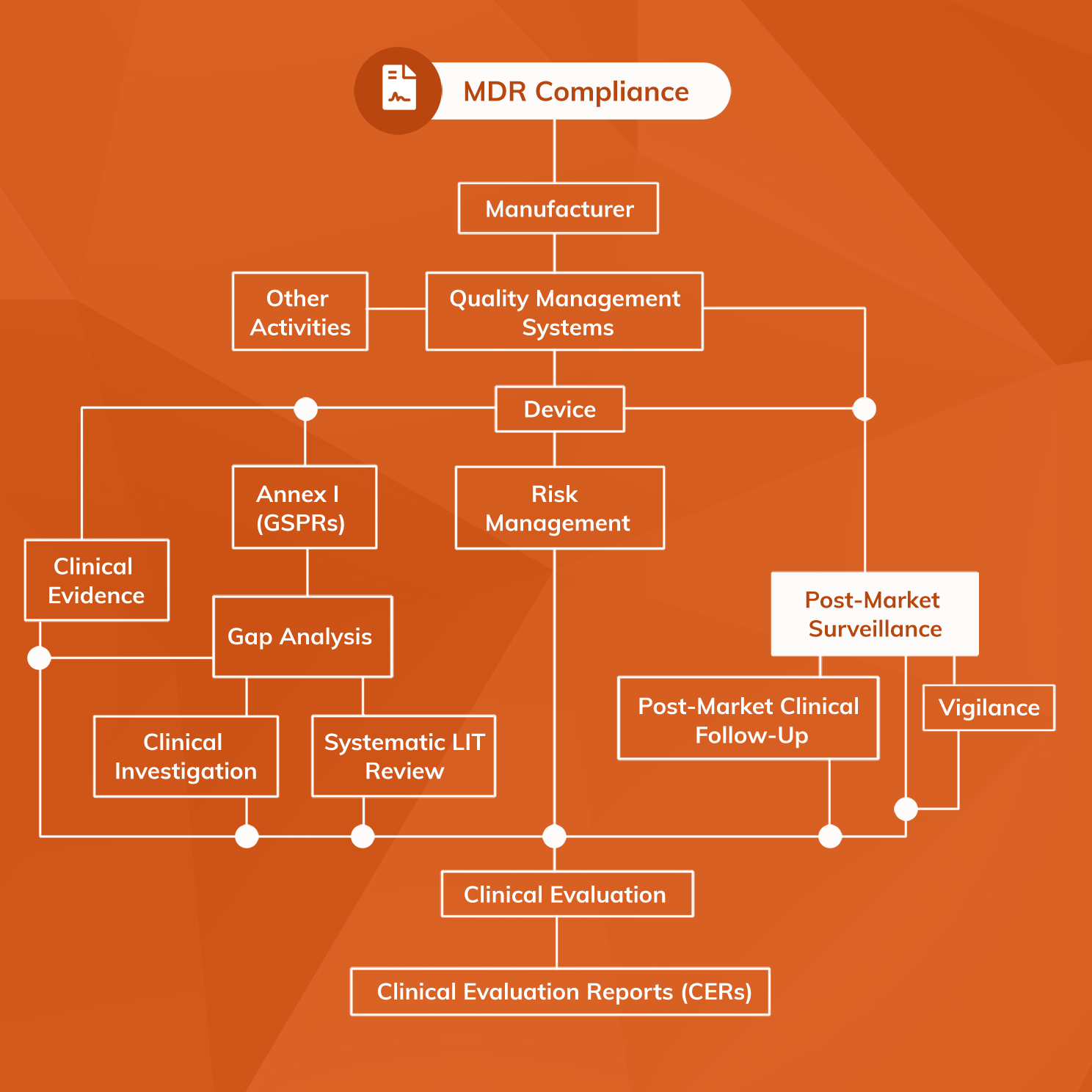
Post Market Surveillance Plan Template prntbl concejomunicipaldechinu
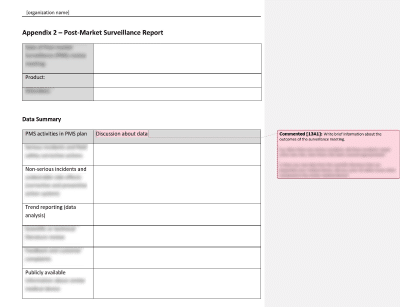
Post Market Surveillance Plan Template prntbl concejomunicipaldechinu

Post Market Surveillance Plan Template prntbl concejomunicipaldechinu
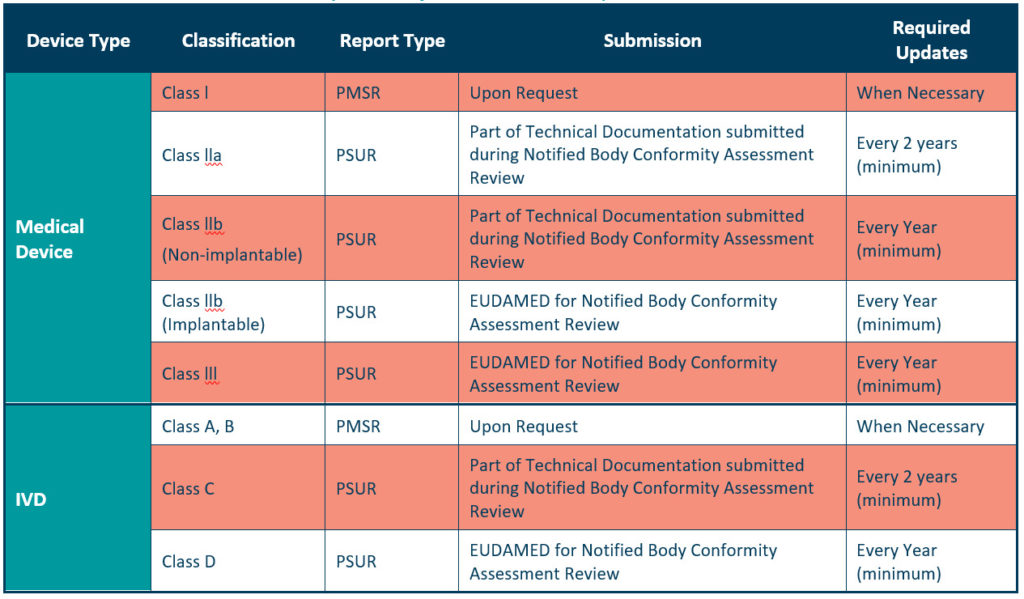
Post Market Surveillance Plan Template prntbl concejomunicipaldechinu
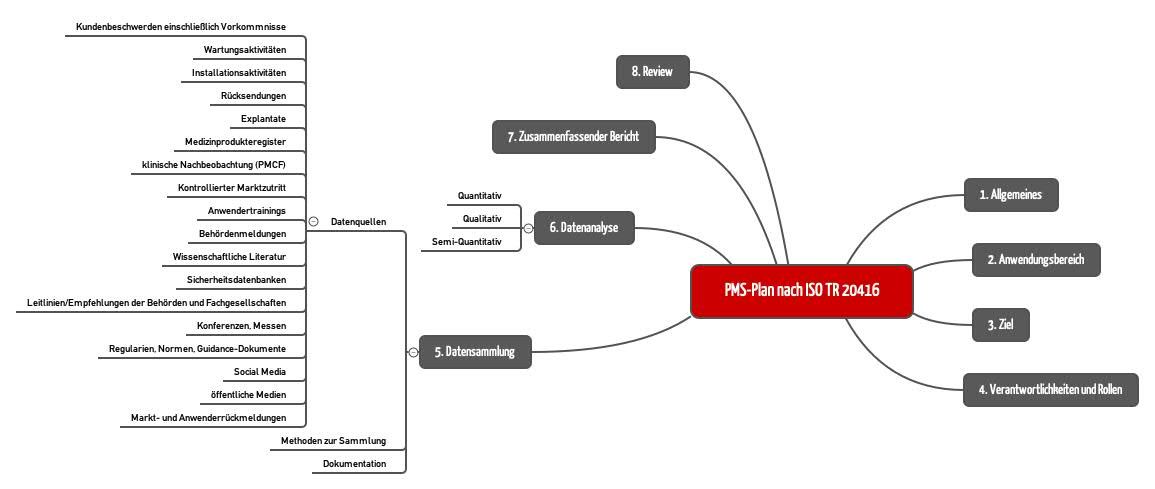
Post Market Surveillance Plan Template prntbl concejomunicipaldechinu

Post Market Surveillance Plan Template prntbl concejomunicipaldechinu
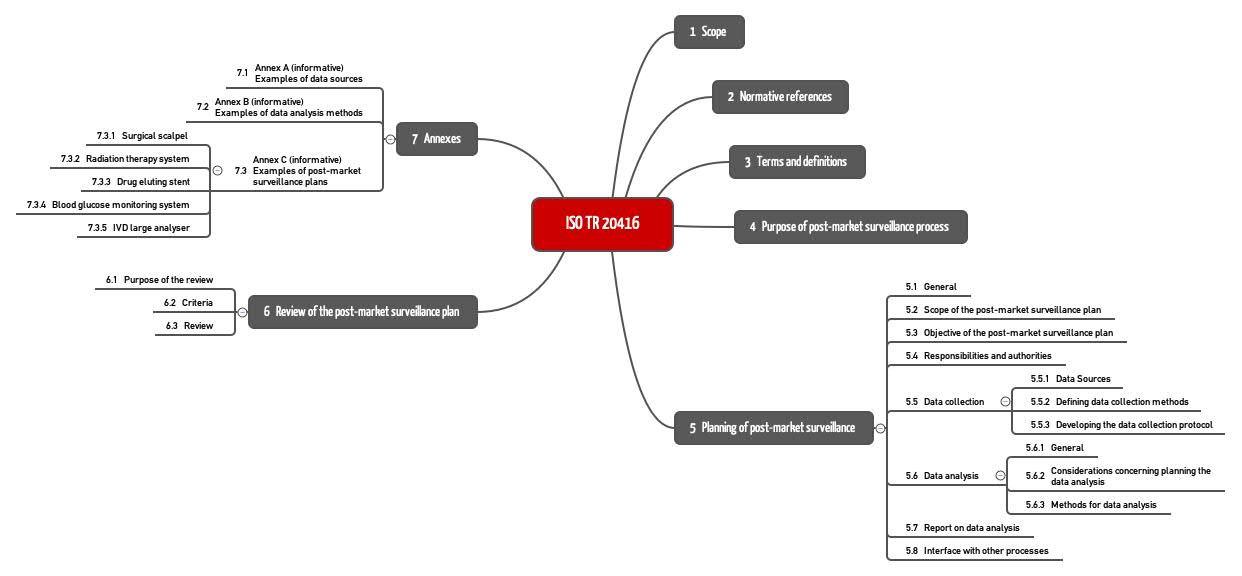
Post Market Surveillance Plan Template prntbl concejomunicipaldechinu

Post Market Surveillance Plan Template prntbl concejomunicipaldechinu

Post Market Surveillance Plan Template prntbl concejomunicipaldechinu

Post Market Surveillance Plan Template prntbl concejomunicipaldechinu

Post Market Surveillance Plan Template prntbl concejomunicipaldechinu

Post Market Surveillance Plan Template prntbl concejomunicipaldechinu

Post Market Surveillance Plan FDA OMC Medical

Post Market Surveillance Plan Template prntbl concejomunicipaldechinu
Post Market Surveillance Plan Template prntbl concejomunicipaldechinu

Post Market Surveillance Plan Template prntbl concejomunicipaldechinu
Post Market Surveillance Plan Template prntbl concejomunicipaldechinu

Post Market Surveillance Plan Template prntbl concejomunicipaldechinu

Post Market Surveillance Plan PMS Plan Template

Post Market Surveillance Plan Template for the MDR DistillerSR

Post Market Surveillance Plan Template for the MDR DistillerSR

Post Market Surveillance Plan Template for the MDR DistillerSR

Post Market Surveillance Plan Template for the MDR DistillerSR

Post Market Surveillance Plan Template for the MDR DistillerSR
Post Market Surveillance Plan Template for the MDR DistillerSR

Post Market Surveillance Plan Template for the MDR DistillerSR

Post Market Surveillance Plan Template for the MDR DistillerSR

PMS Plan Post Market Surveillance Plan Template
Post Market Surveillance Plan Template for the MDR DistillerSR
Post Market Surveillance Plan Template for the MDR DistillerSR

Postmarket Surveillance Process Procedure 4EasyReg

Post Market Surveillance Plan 2 PDF Medical Device Myopia
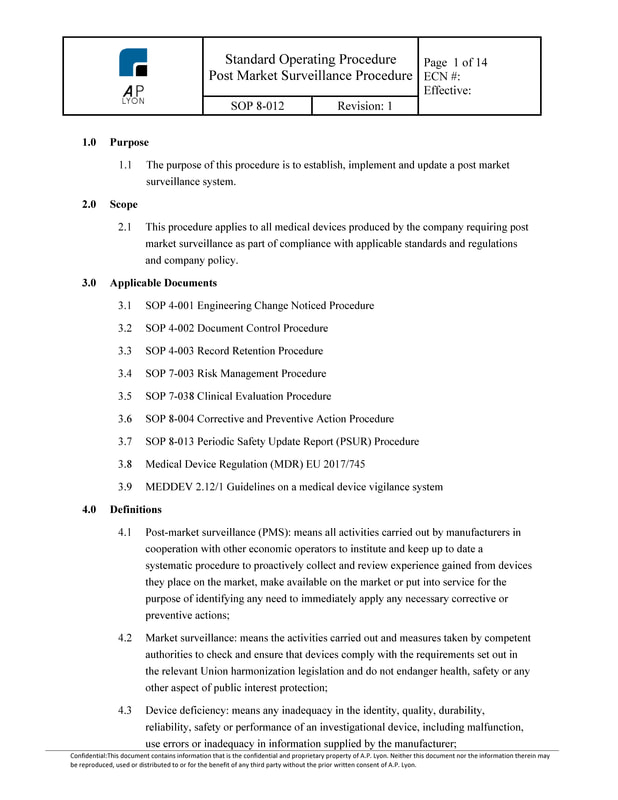
Post Market Surveillance Procedure

Medical Device Post Market Surveillance (PMS)

Reporting post market surveillance activities Medical Device HQ 1

Post Market Surveillance Plan: the process of surveillance on the

2024 EU MDR/IVDR Guide: Post market Surveillance Casus Consulting

Postmarket Surveillance Plan Template 4EasyReg

Postmarket Surveillance Plan Template 4EasyReg

How Post Market Surveillance Enhances Medical Device Safety PlatoHealth
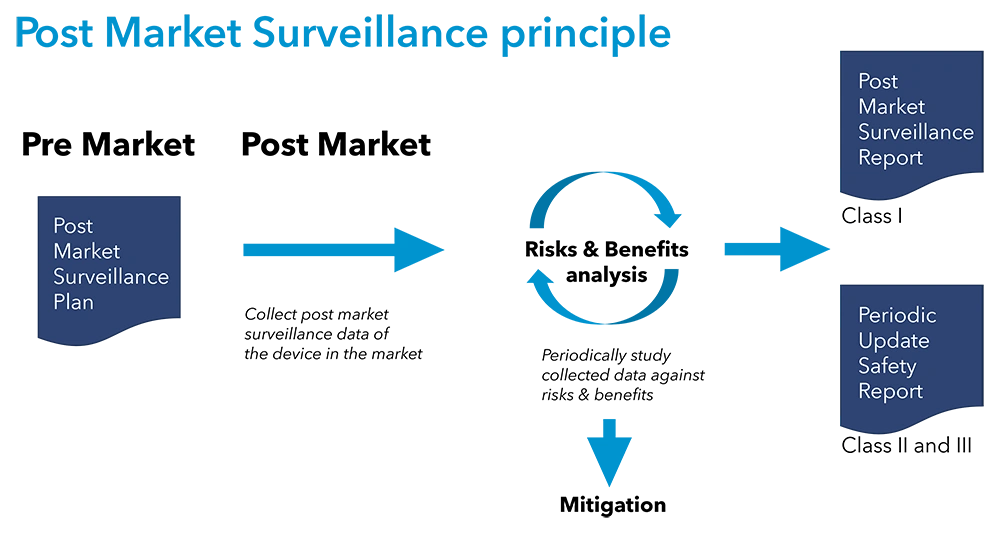
Post Market Surveillance with BAAT Medical

How to Set Up a Post Market Surveillance System specculo

How to Set Up a Post Market Surveillance System specculo

Post Market Surveillance for Medical Devices: Ensuring Safety and