Medical Device Quality Plan Template
Here are some of the images for Medical Device Quality Plan Template that we found in our website database.

Medical HD Wallpapers Top Free Medical HD Backgrounds WallpaperAccess

MEDICAL EQUIPMENT Wallpapers Wallpaper Cave

Medical Wallpaper
Medical Tourism In Eastern Europe

Healthcare in India Sattva Consulting

10 things you didn t know about medical residents AAMC

Medical Diagnosis
3d medical sign symbol concept icon 21658602 PNG

The Medical Industry: The Future of Healthcare Wellness

Discover the Latest Advances in Medical Technology PaxeraHealth

Benefits of AI in Healthcare

What to look for when taking out medical aid

Medical Doctor Symbol ClipArt Best

Surgery Claims Medical Law

FREE Medical Symbol Caduceus Images ClipArt Best
Should medical tourism be used to supplement gaps in our health care

Three Common Questions in a Medical Malpractice Case

Medical Imaging Diagnostics at Bradley Briseno blog
Medical Examination: Định nghĩa Cách sử dụng và Các cụm từ liên quan
_1662041110.png)
Decoding The Future Of Medical Technology Skill Lync Blogs
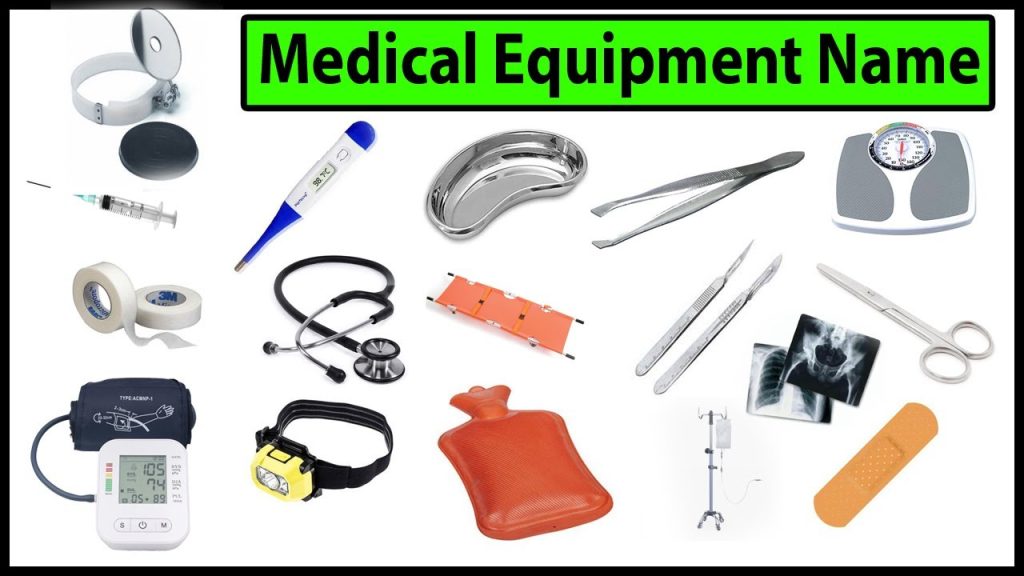
Basic Medical Equipment List Required in a Hospital Health Tips

What is Medical Terminology? Explanations Helpful Resources

Medical Terminology For Healthcare Professionals 10th Editio
Free Medical Infographic Design Dribbble Graphics

Medical Information Peermed

Navigating the Labyrinth: Your Guide to Smart Medical Care Saved By

Medical Report What Is a Medical Report? Definition Types Uses

Medical Coding: A Critical Role in Healthcare Today

All about the Pharmaceutical and Medical Device Industry Infographic

Medical Terminology: Learning Through Practice
25 Inspiring Medical Website Design Examples (With Tips) Magezon

How U S medical practices can help us all get healthier

Medical Raphael Health Center

Medicine Symbol ClipArt Best
Medical Services Orange Blossom Family Health