Medical Device Cybersecurity Certification
Here are some of the images for Medical Device Cybersecurity Certification that we found in our website database.

Medicine Photos Download The BEST Free Medicine Stock Photos HD Images
COVID 19: How innovations in health technology can help Nigeria

10 things you didn t know about medical residents AAMC
100 Hd Medical Wallpapers Wallpapers com

Healthcare in India Sattva Consulting

Medical Diagnosis
Medical Tourism In Eastern Europe
What Is A Medical Device Fda Definition at Theresa Ward blog

Imaging

HD Medical Wallpapers Top Free HD Medical Backgrounds WallpaperAccess

Day in the Life of a Medical Student: Clinicals Blueprint Prep
_1662041110.png)
Decoding The Future Of Medical Technology Skill Lync Blogs

4 Reasons to Implement Healthcare Cloud for Digital Transformation
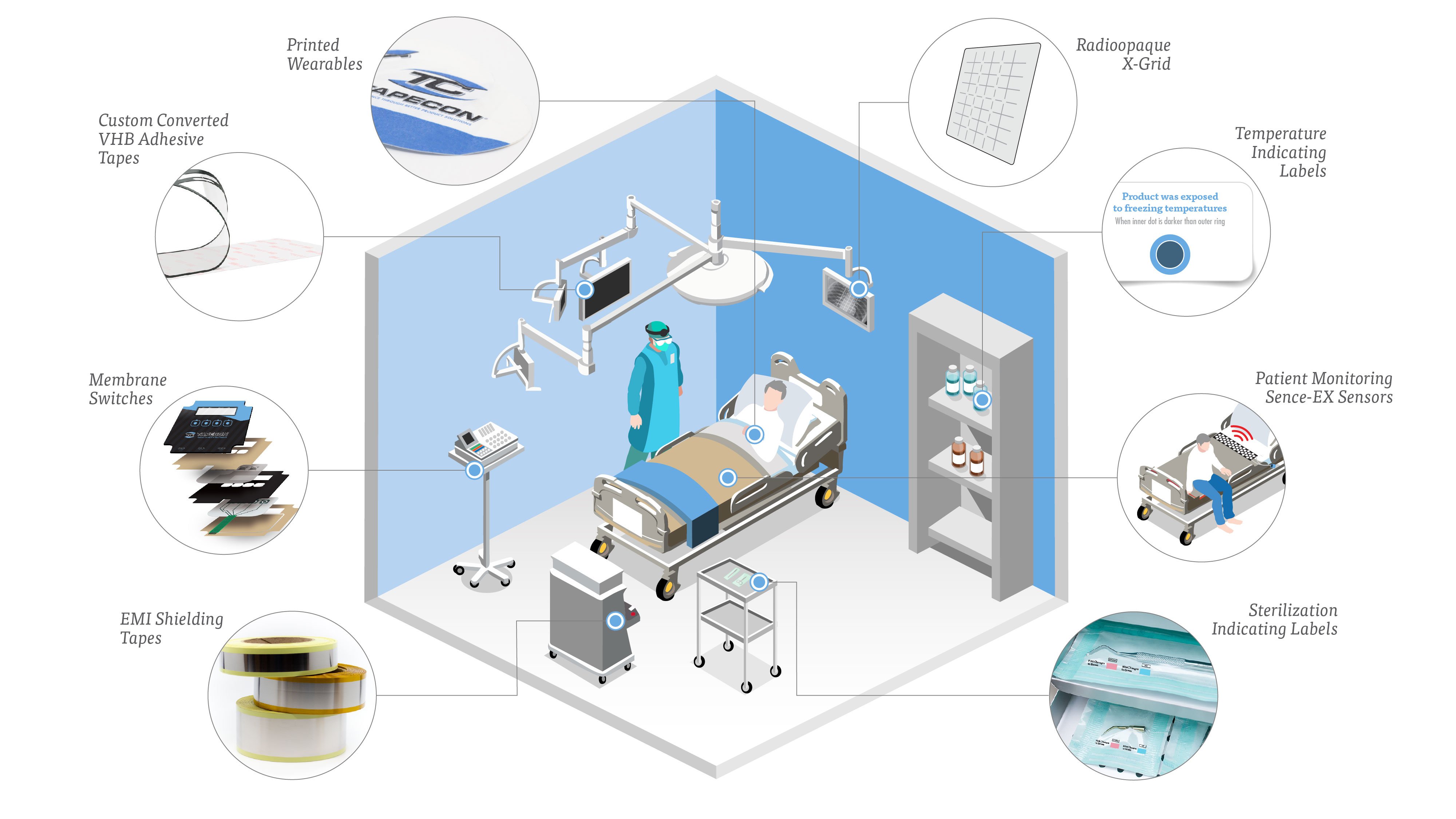
Healthcare and Life Sciences Solutions Tapecon

National Medical Devices Policy Approved By Union Cabinet

5 Use Cases Of AI powered Medical Imaging In Healthcare

How to Become a Medical Research Scientist as a Premed Shemmassian

Top tips for teaching and learning from one of Canada s best

Medical Exams l Glendale CA l JoinGPD

8 medical advances you may have missed during COVID 19 AAMC

Virtual Reality Training for Healthcare: The New Tool in Medical Education

Medical Items Vocabulary ll 130 Medical Items Name In English With

Medical Raphael Health Center

Medical Laboratory Science Job Description Roles/Responsibilities and
Should medical tourism be used to supplement gaps in our health care

What is Medical Terminology? Explanations Helpful Resources

Internal Medicine Doctor in Sheepshead Bay Brooklyn
CONTACT PrimeCare Medical Centre and Bowmanville Medical Centre

Medical Doctor Symbol ClipArt Best

Medicine Symbol ClipArt Best

Medical Information Peermed

FREE Medical Symbol Caduceus Images ClipArt Best

Optimizing the healthcare supply chain McKinsey

Surgery Claims Medical Law

Medical Report What Is a Medical Report? Definition Types Uses
3d medical sign symbol concept icon 21658602 PNG
/where-healing-happens-844399628-5a2abb2feb4d52003669ac10.jpg)
The Basics of Major Medical Health Insurance
Medical Services Orange Blossom Family Health

Medical Billing Coding Duties Job Description SCI
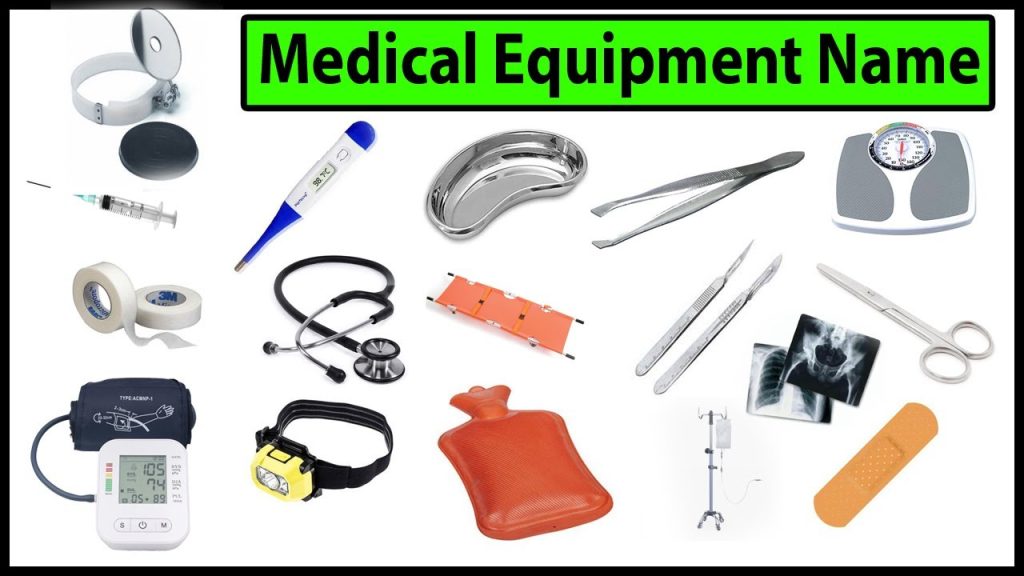
Basic Medical Equipment List Required in a Hospital Health Tips

What to look for when taking out medical aid

15 Major Components of a Complete Medical Record

Best New York Medical and Diagnostic Services Center NYMDC
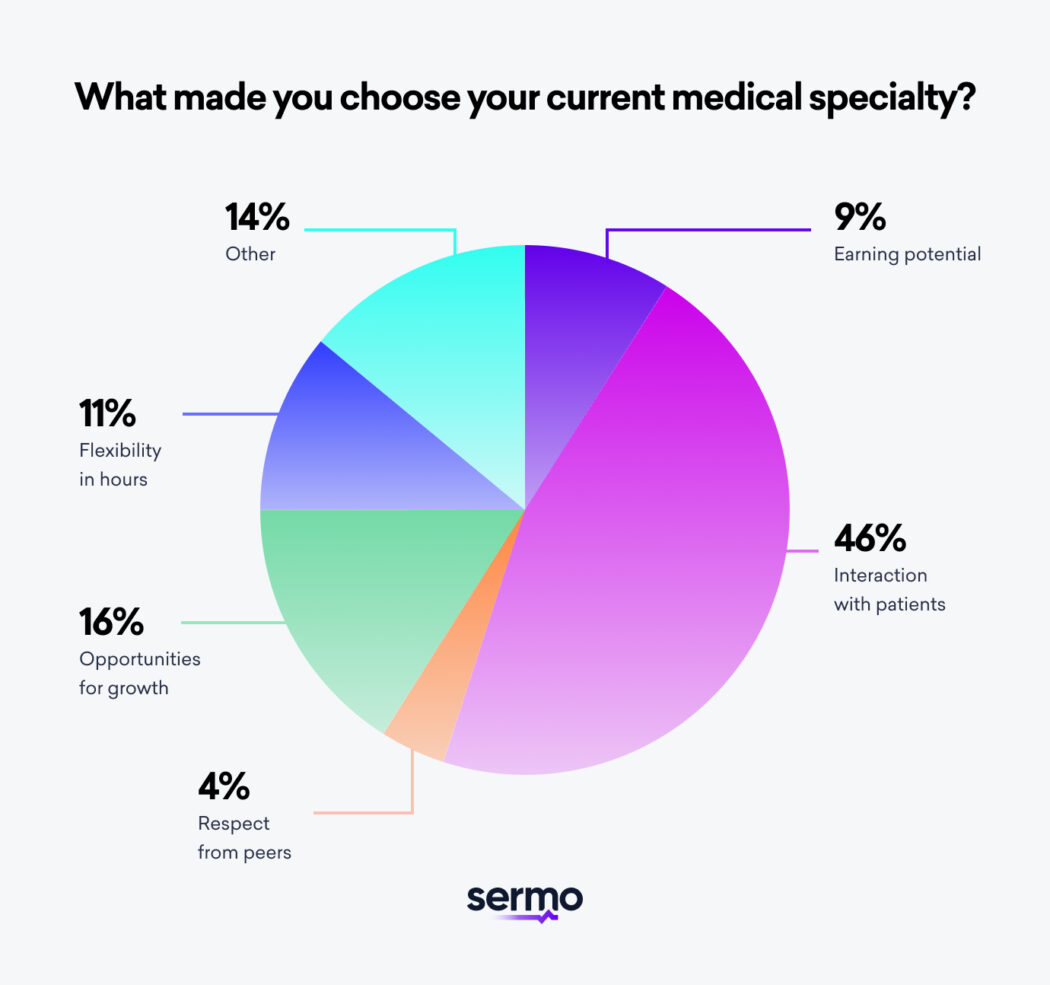
Physician s Guide to the Best Medical Specialties Sermo

60 Medical Equipments List of Hospital Equipments Medical
25 Inspiring Medical Website Design Examples (With Tips) Magezon
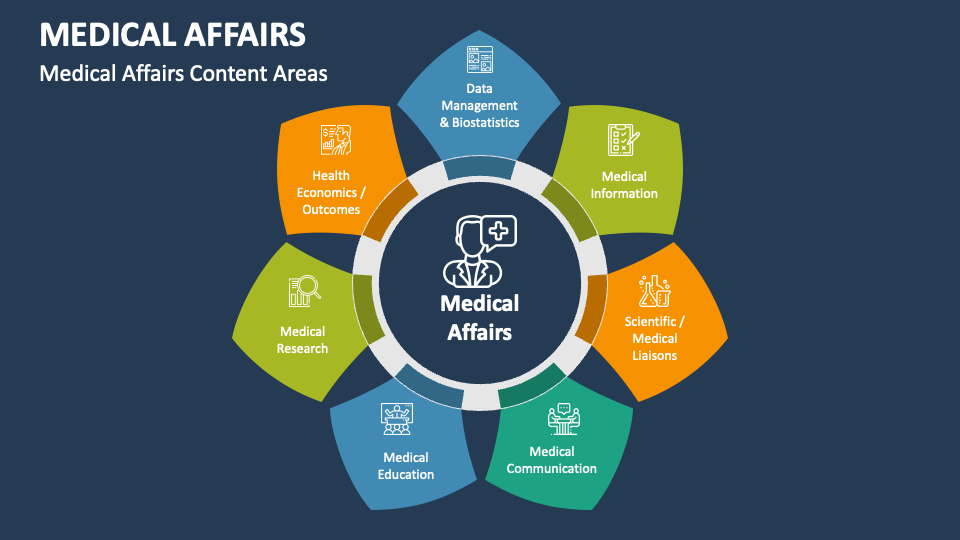
Medical Affairs PowerPoint Presentation Slides PPT Template

Medical app Figma
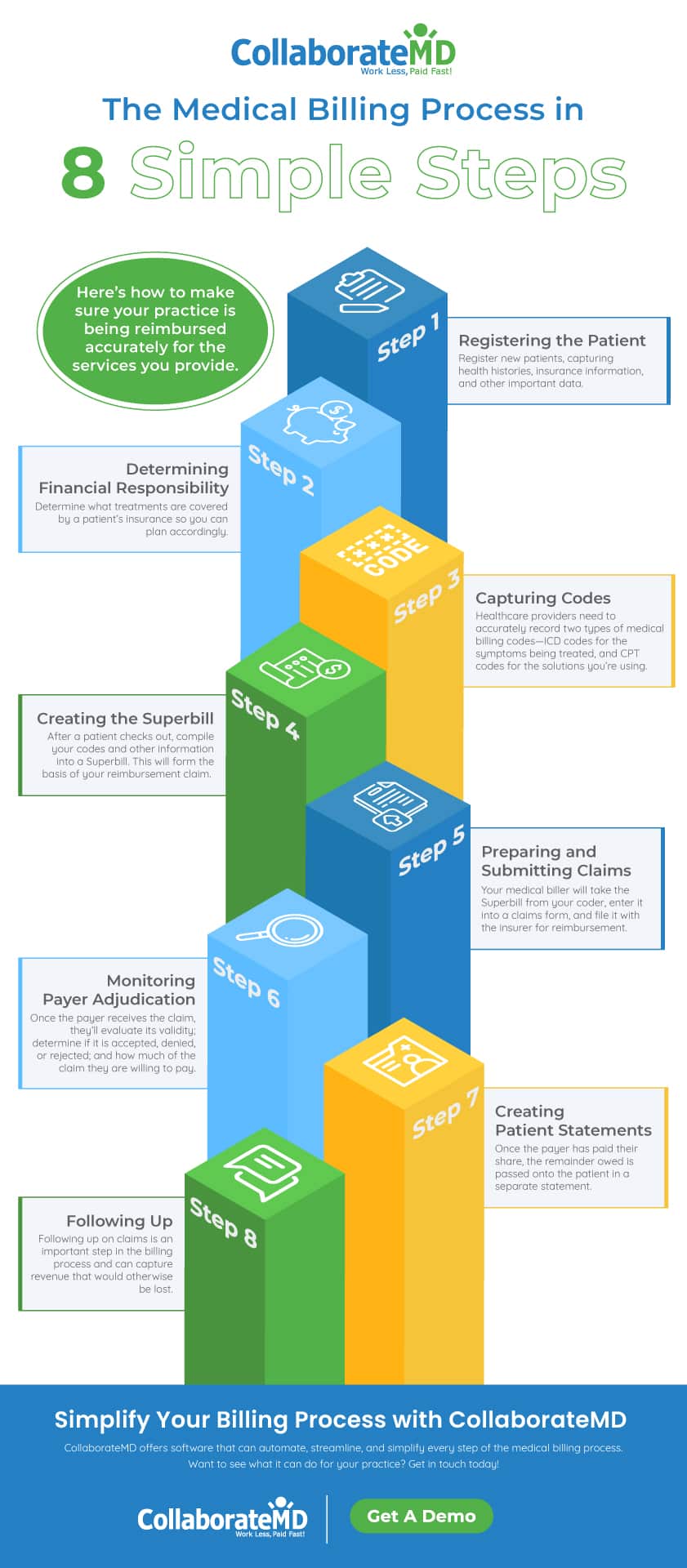
What Is the Medical Billing Process? Steps Cycle Overview
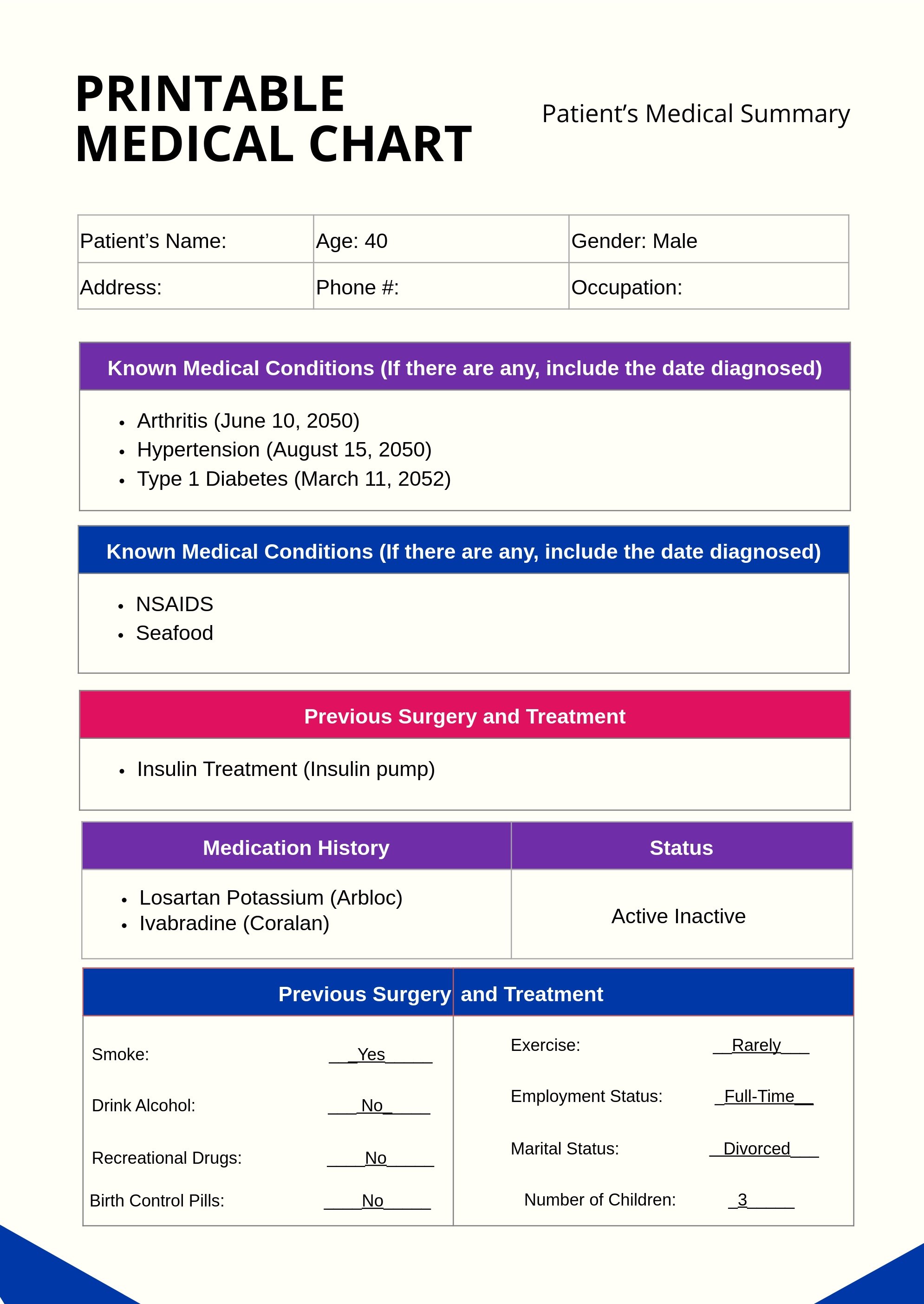
FREE Medical Chart Templates Examples Edit Online Download

Medical Illustrations Solution ConceptDraw com

Medical Terminology List Printable Pdf Medical Prefixes and Suffixes
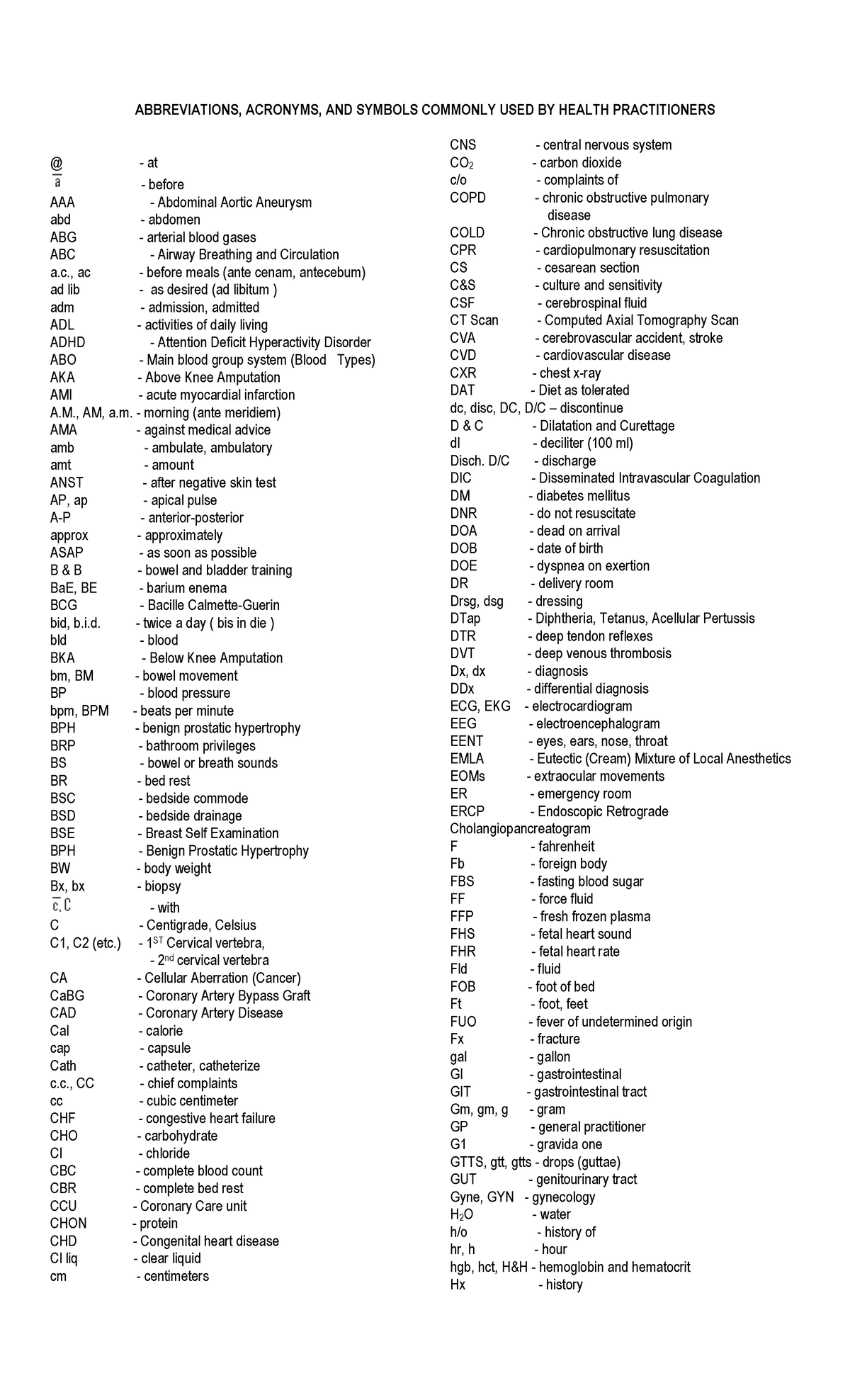
Common Medical Abbreviations ABBREVIATIONS ACRONYMS AND SYMBOLS
Medical Terminology Series Eastern Mennonite University
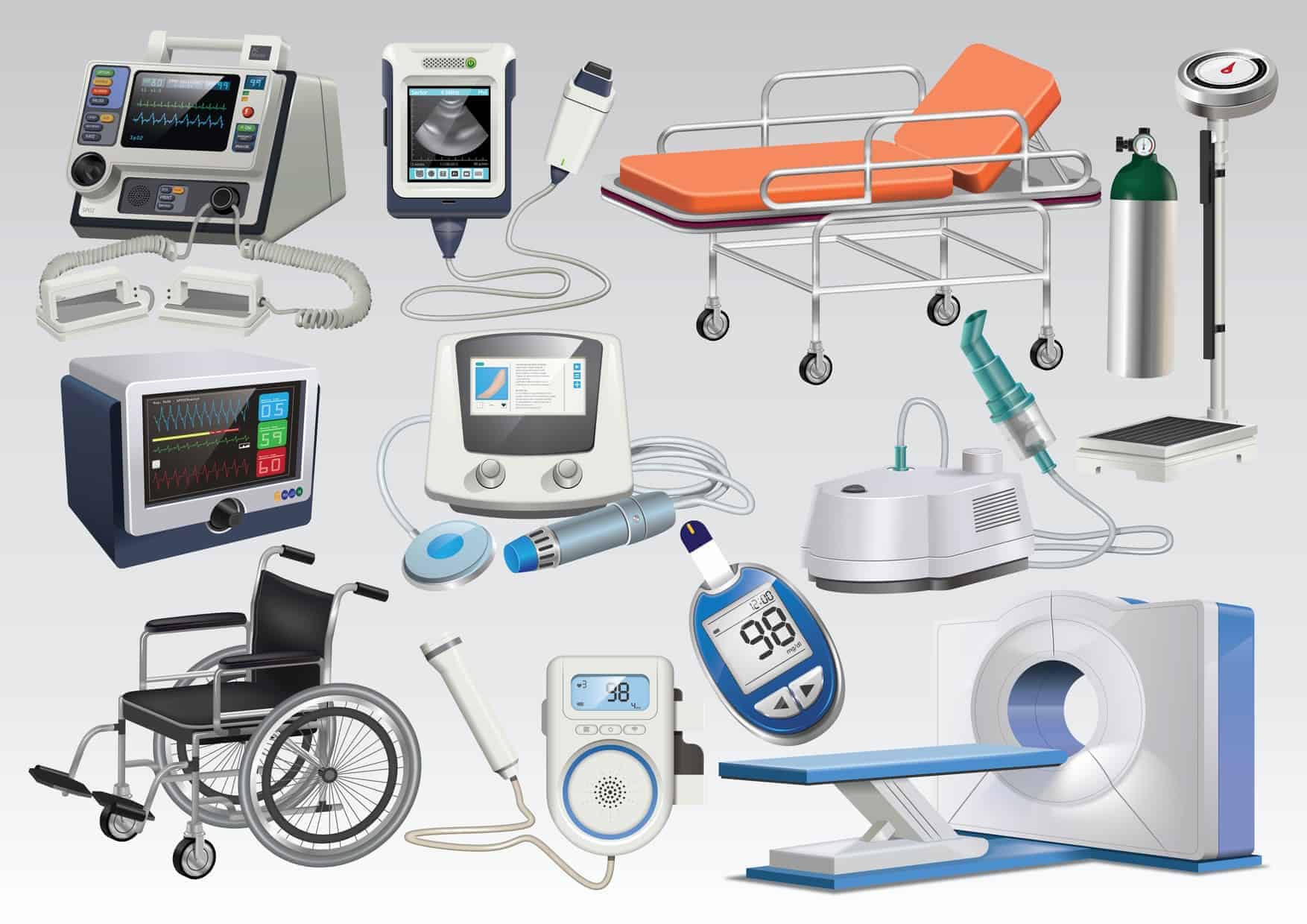
How Do You Know Home Medical Equipment Is Certified? SonderCare

Basics Of Medical Terminology
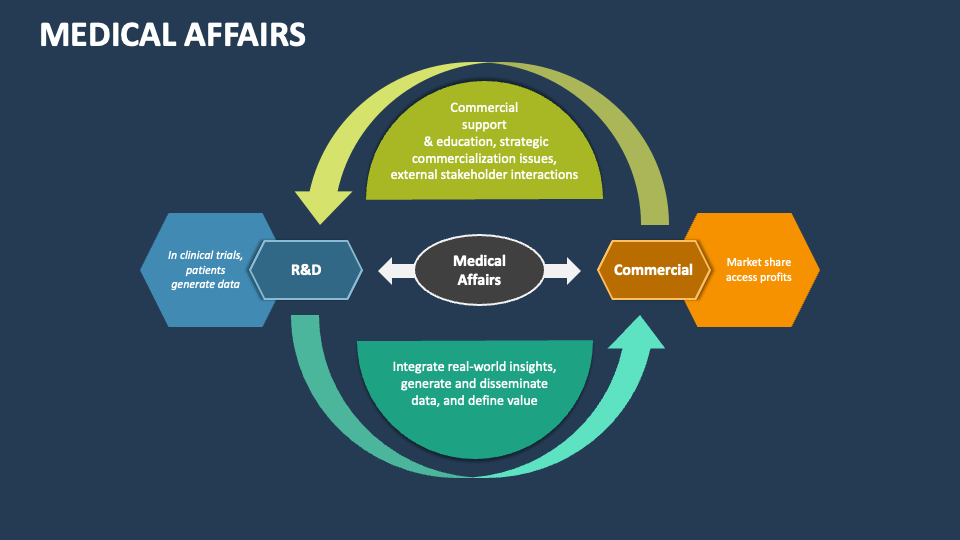
Medical Affairs PowerPoint Presentation Slides PPT Template

How U S medical practices can help us all get healthier

How Can Hospitals Make Patient Care and Medical Info Work Together

New Medical Records Keeping Course Available Online College News