Gxp Process Validation Certification
Here are some of the images for Gxp Process Validation Certification that we found in our website database.
Certified and CPD/CEU Accredited Trainings in Regulatory Affairs

Certified and CPD/CEU Accredited Trainings in Regulatory Affairs

ValGenesis Launches Smart GxP™ to Unify Validation Process

ValGenesis Launches Smart GxP™ to Unify Validation Process
GxP Engagement: Aseptic Process and Equipment Validation i Pharm

GxP Engagement: Aseptic Process and Equipment Validation i Pharm

Process validation from planning to production

Harness the Power of AI in GxP Use Cases Process Optimization and

Computer System Validation (CSV) Online Course And Certification

Computer System Validation (CSV) Online Course And Certification

Computer System Validation (CSV) Online Course And Certification
Computer System Validation (CSV) Online Course And Certification

Computer System Validation (CSV) Online Course And Certification

Validation Mapping and other Services for GxP Regulated Applications PST

Gxp Compliance and Validation On going Paperless

Gxp Services BSAssociates

Software Validation Online Course And Certification

Software Validation Online Course And Certification

GxP Infrastructure Qualification Installation Qualification

What is GxP in pharma?

Guide to GxP compliance: processes challenges and tools

What is GxP? Dassault Systèmes blog

Good Distribution Practice (GDP) Online Training and Certification

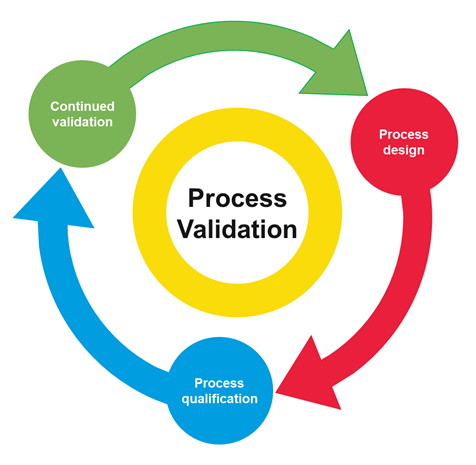
What is Process Validation in the Pharma Industry Sware

What is Process Validation in the Pharma Industry Sware

What is Process Validation in the Pharma Industry Sware

Quality System Control Management Service Validation Process Stock
Molding Process Validation In Ensuring Quality Consistency VolksMolds

Sarwar Ali on LinkedIn: Medical Device Process Validation ISO 13485
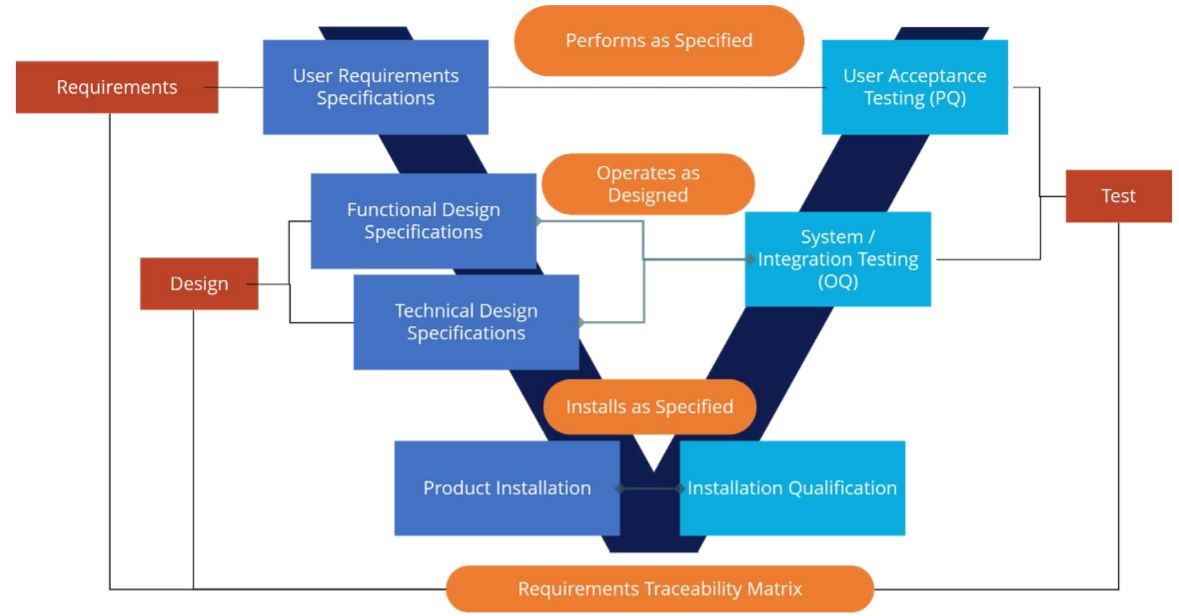
Blog: Optimizing GxP Compliance Costs in Life Sciences

Computer System Validation GxP process Owner and Quality Assurance

About Us ProcessX
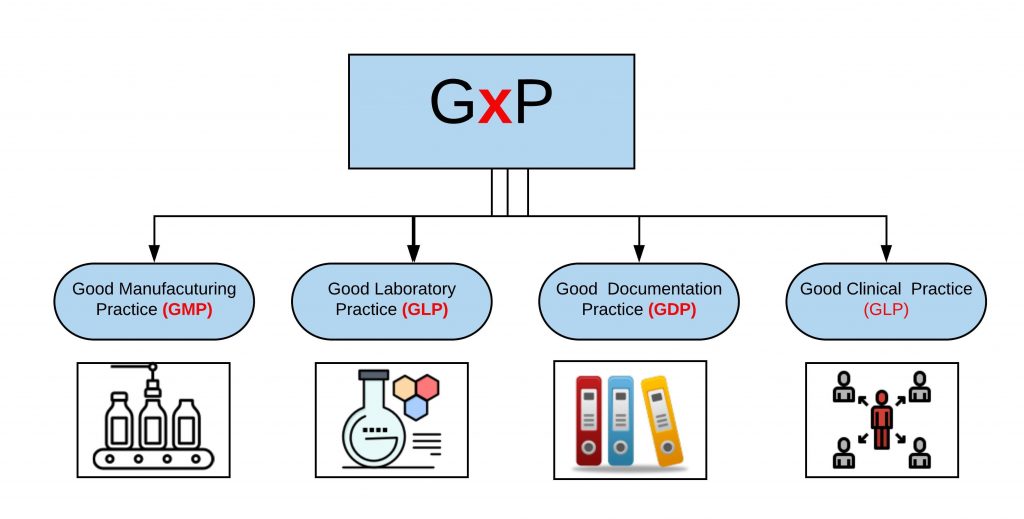
Images of GxP JapaneseClass jp

Computer System Validation

Guide to the Verification and Validation Methodology (V Model)
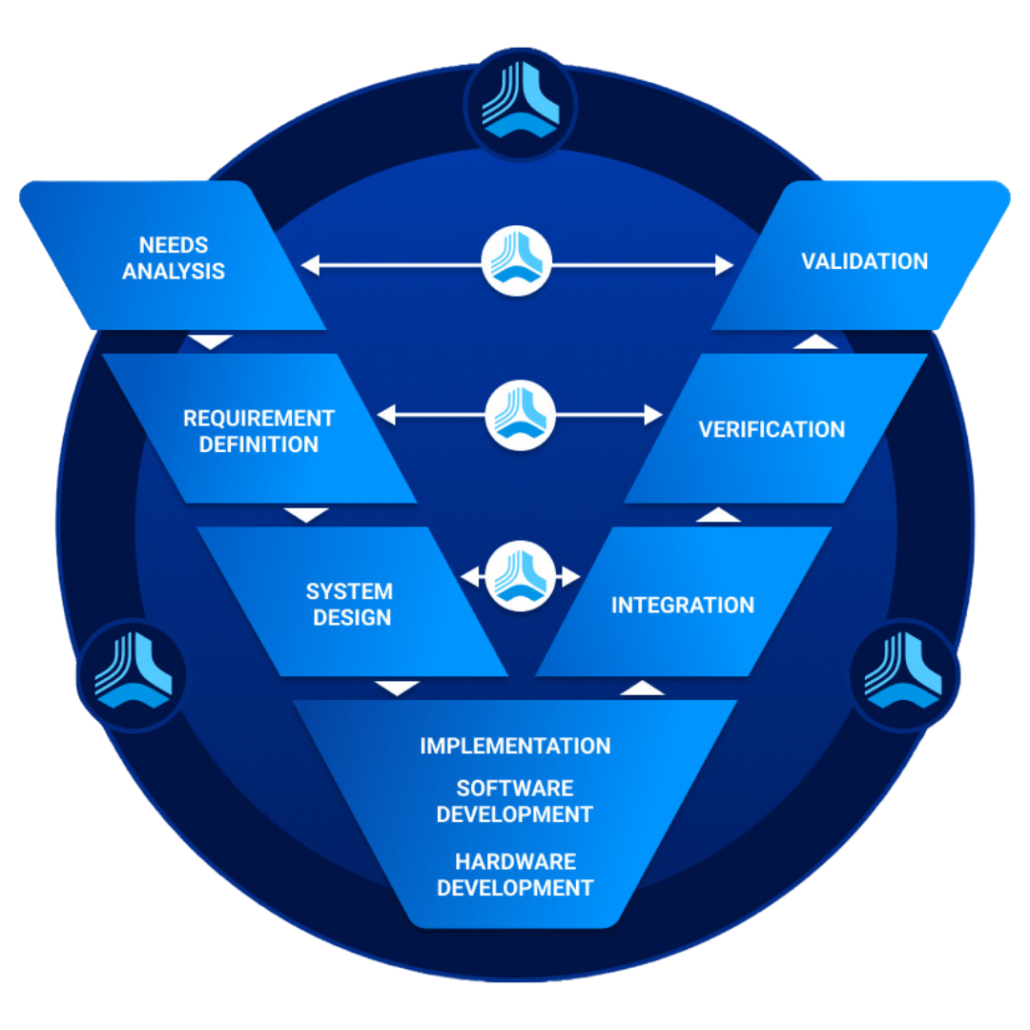
Best Practices for Verification and Validation Jama Software
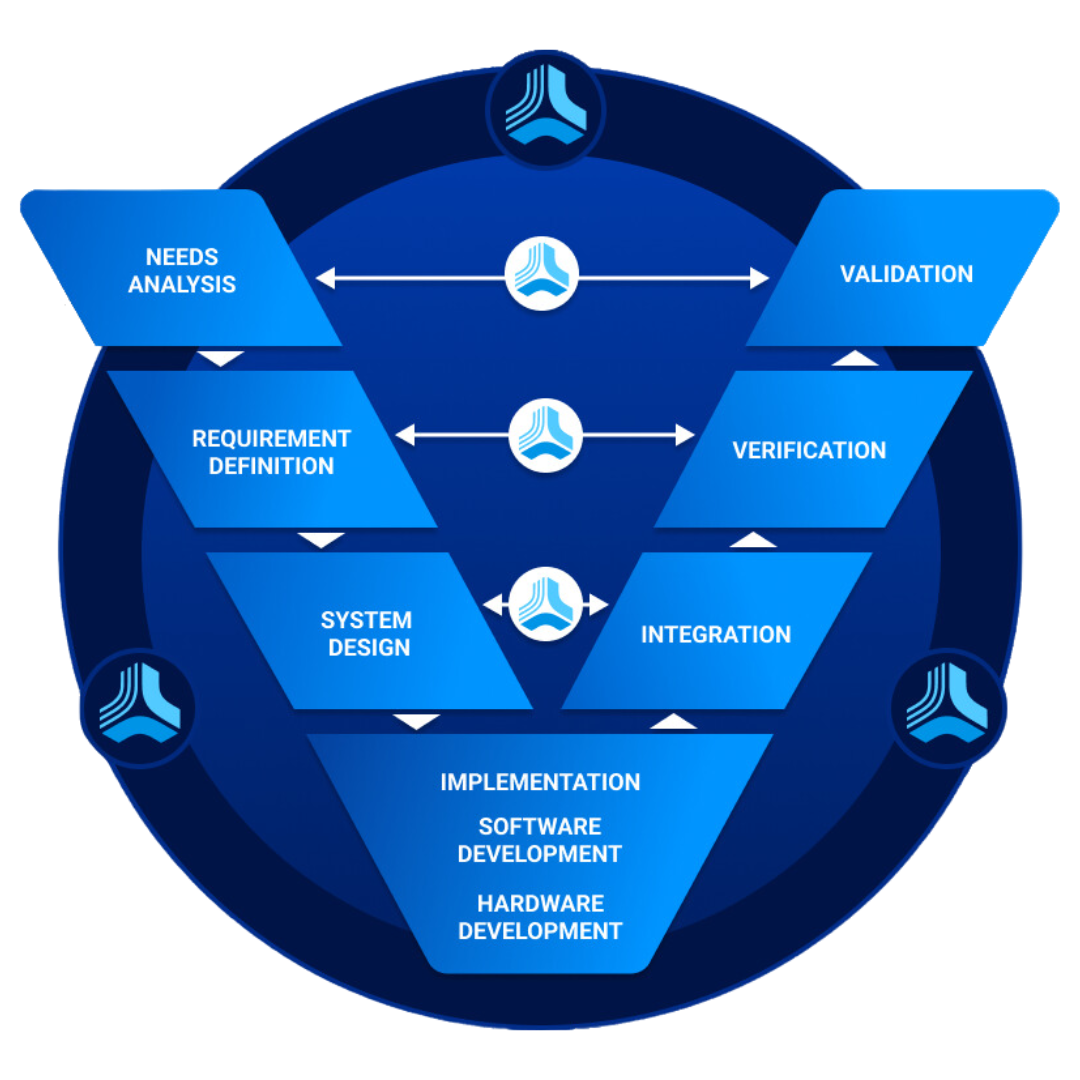
Best Practices for Verification and Validation Jama Software

Drug Safety Reporting and AE Data Reconciliation: Two systems and processes

Accueil GxP Conseils
_00010.jpg)
GxP Equipment Qualification Process Valsyner Consultancy

Survey Request Email Template prntbl concejomunicipaldechinu gov co

SUSAR: How can they be defined
VETTER: 4 MAIN CHARACTERISTICS OF LEARNING PROCESSES IN FORMAL NON

David Lai Director of Quality Compliance and Excellence for Life