Fda Pre Submission Template
Here are some of the images for Fda Pre Submission Template that we found in our website database.

FDA Pre Submission Cover Letter PDF Food And Drug Administration

Fda Pre Submission Template
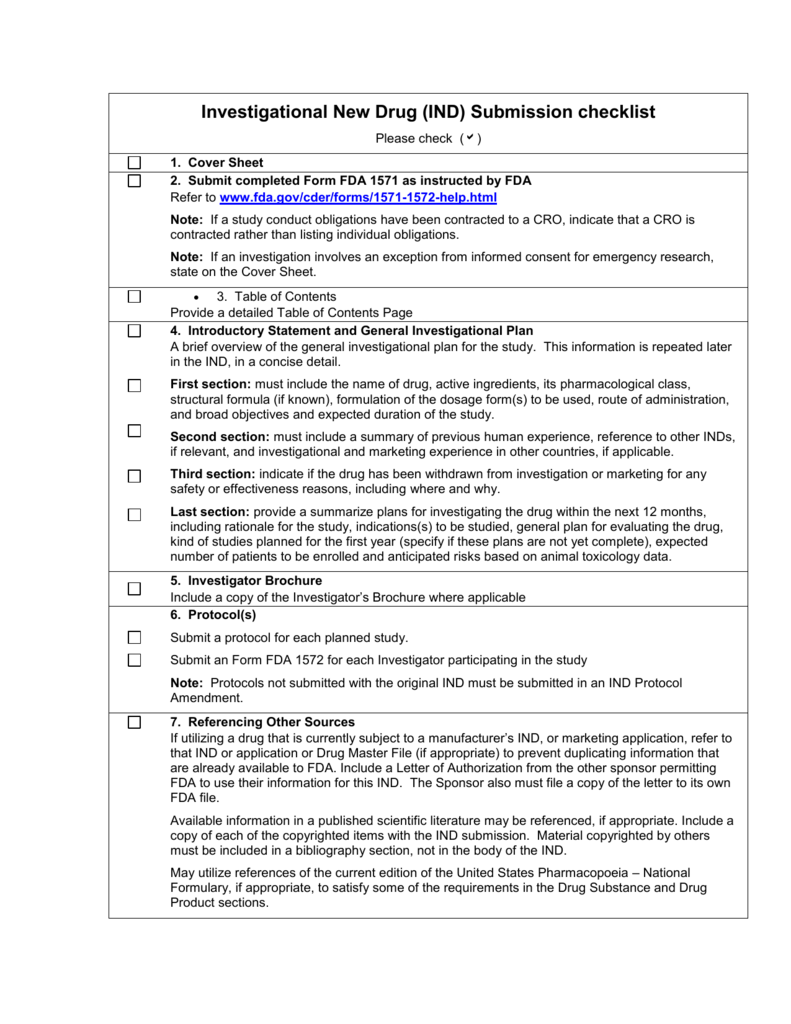
Fda Pre Submission Template
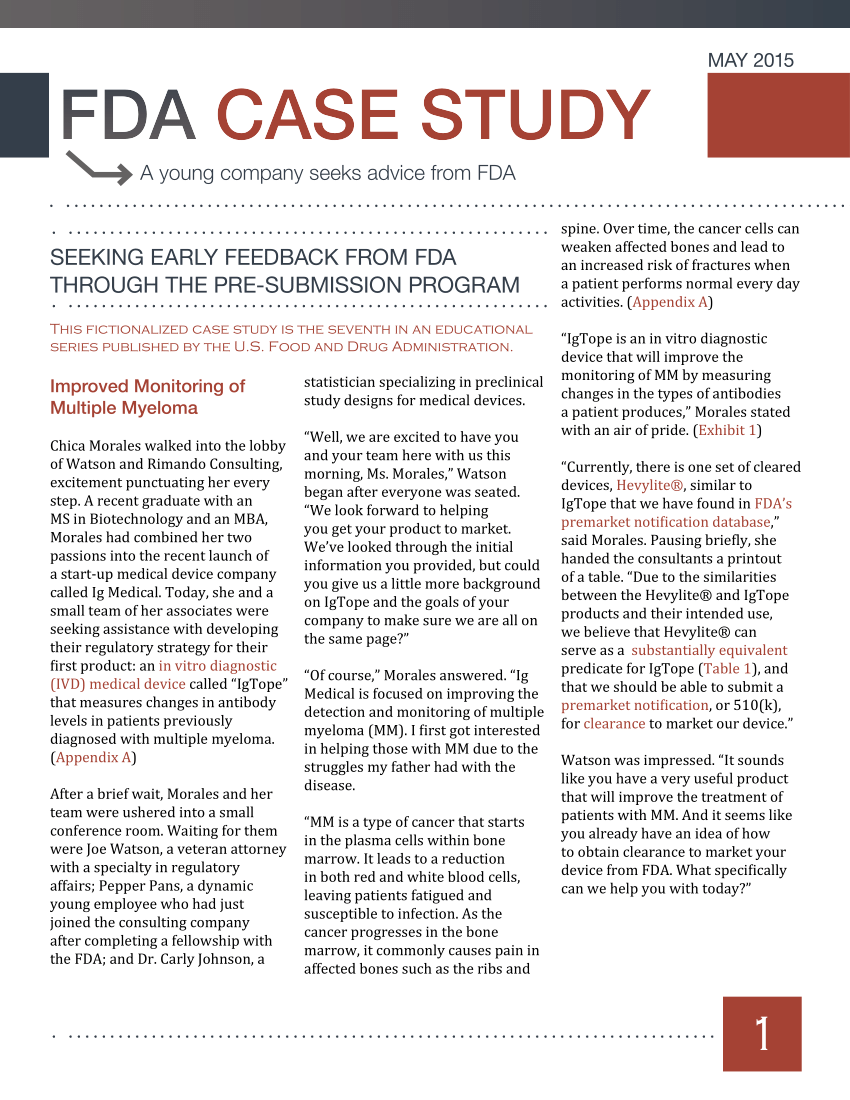
Fda Pre Submission Template
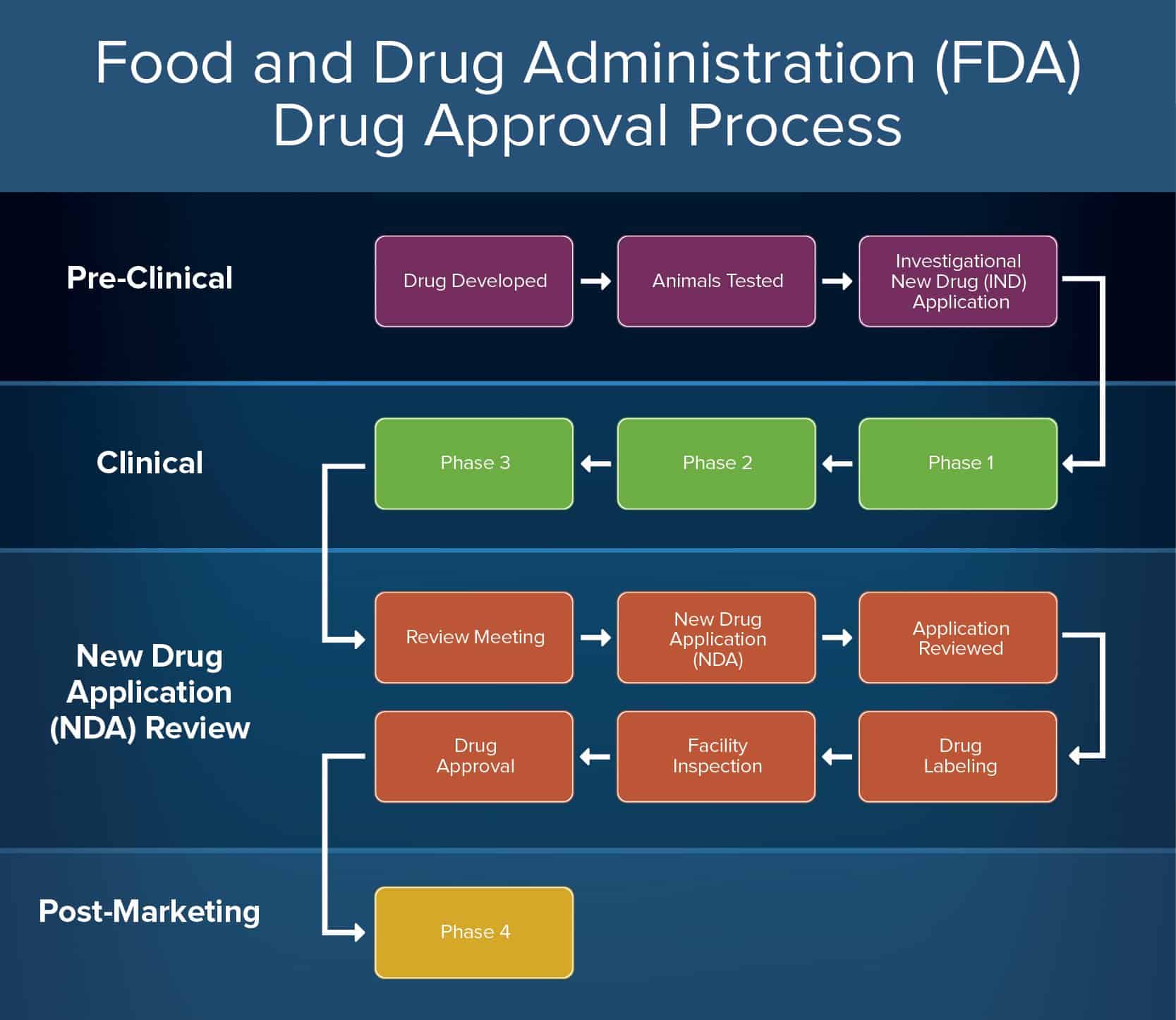
Fda Pre Submission Template

Fda Pre Submission Template

Fda Pre Submission Template

Fda Pre Submission Template

Fda Pre Submission Template

Fda Pre Submission Template
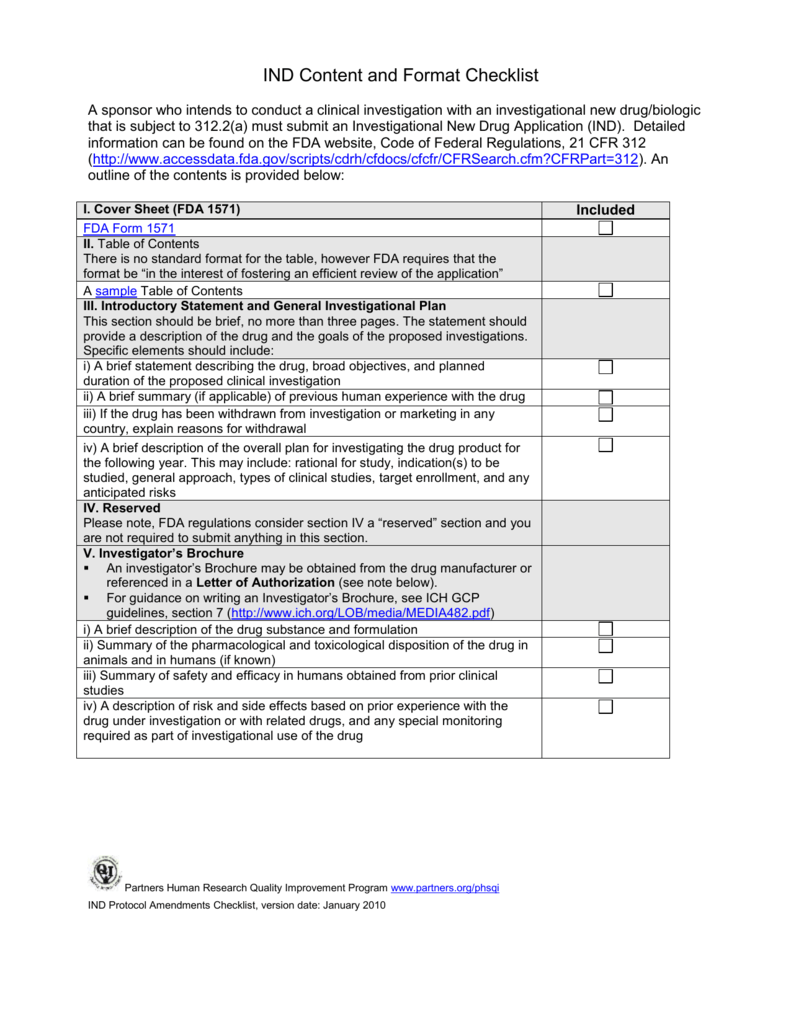
Fda Pre Submission Template

Fda Pre Submission Template

Fda Pre Submission Template

FDA eSTAR Submission Template: A Guide to Navigating the Process
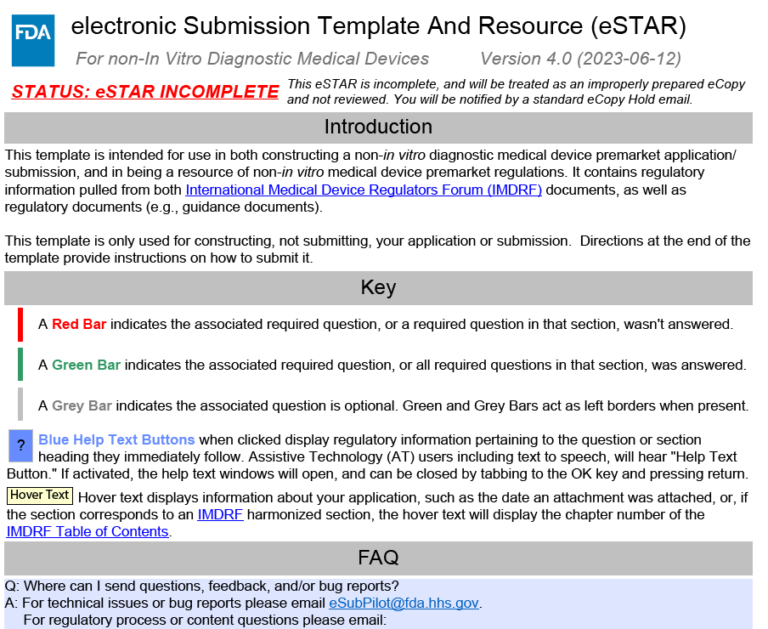
FDA eSTAR Submission Template: A Guide to Navigating the Process
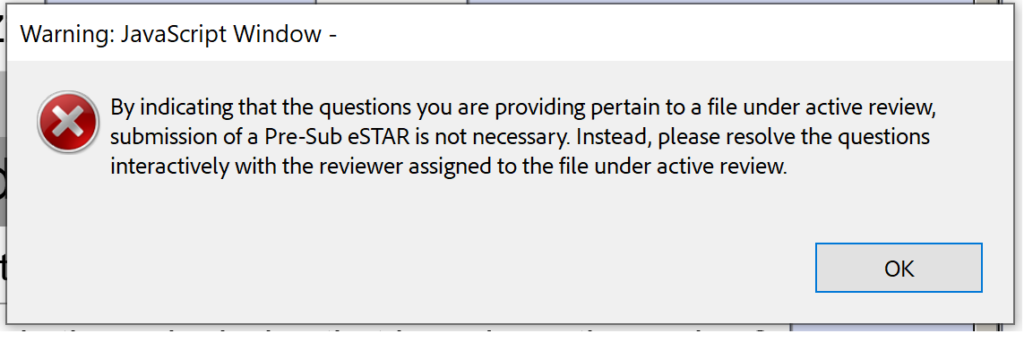
FDA Pre Submission Format and Content Requirements Medical Device Academy

FDA Pre Submission Format and Content Requirements Medical Device Academy

FDA Pre Submission Format and Content Requirements Medical Device Academy
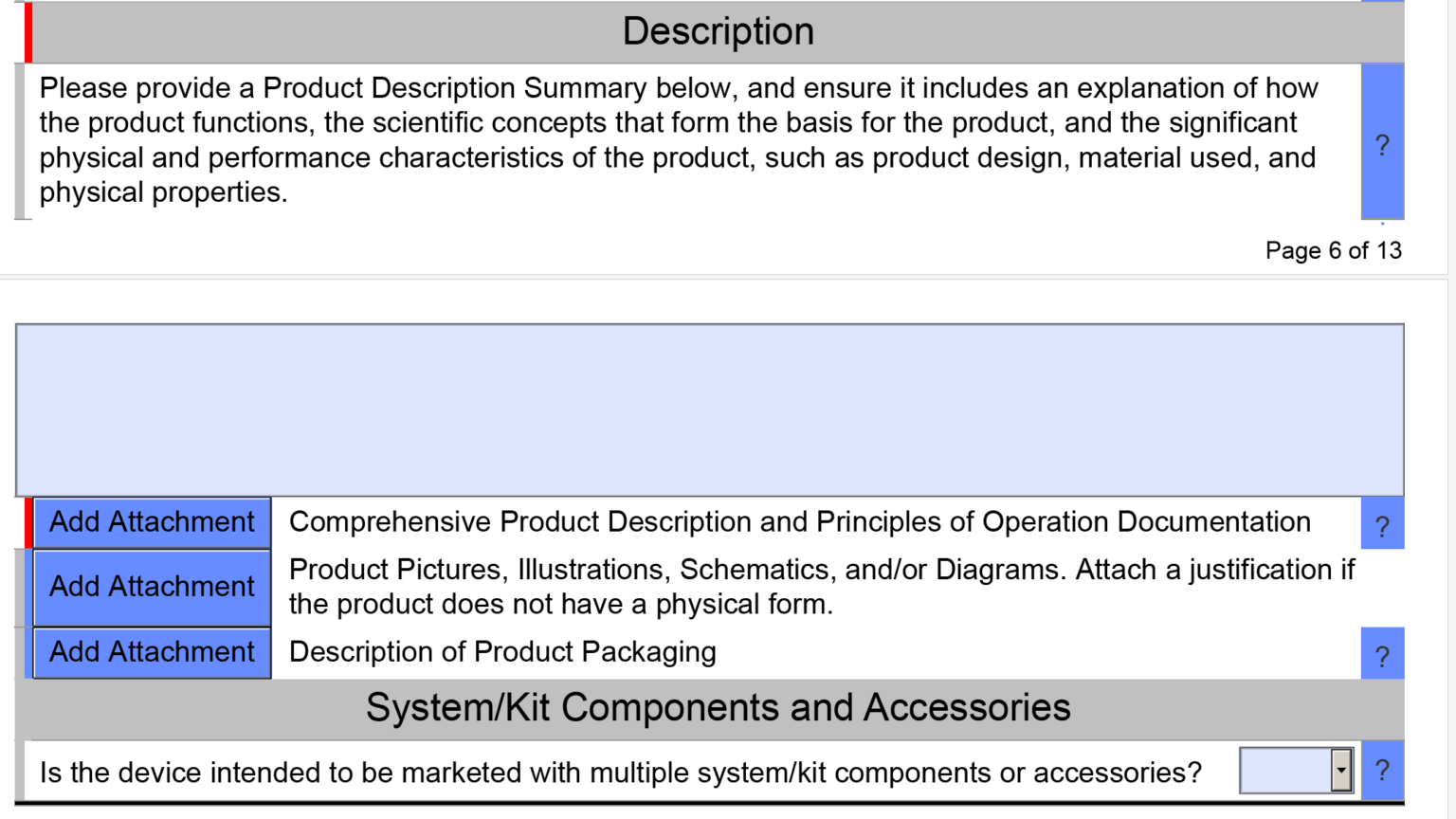
FDA Pre Submission Format and Content Requirements Medical Device Academy

FDA Pre Submission Format and Content Requirements Medical Device Academy

FDA Pre Submission Format and Content Requirements Medical Device Academy

What is a Pre submission (FDA) and why should manufacturers use it

FDA Pre Submission Meeting Checklist for Medical Device Companies

Fda Pre Submission Template Web the estar is an interactive pdf form

A Pre Submission (Q Submission) template for submittal to FDA Upwork

How to Prepare an FDA Pre Submission Free Download

FDA Pre Submission: All You Need to Know Operon Strategist

FDA Pre Submission Support for Medical Devices ADB CRO

FDA eCopy Submission Guide Device Layout Checklist

Redesigned Pre Submission Meetings for ANDAs with the US FDA DDReg Pharma

What Is an FDA Pre Submission (Q Sub)? Complete 2025 Guide

What Is an FDA Pre Submission (Q Sub)? Complete 2025 Guide

What Actually Happens in a Pre Submission Meeting with FDA? Part I
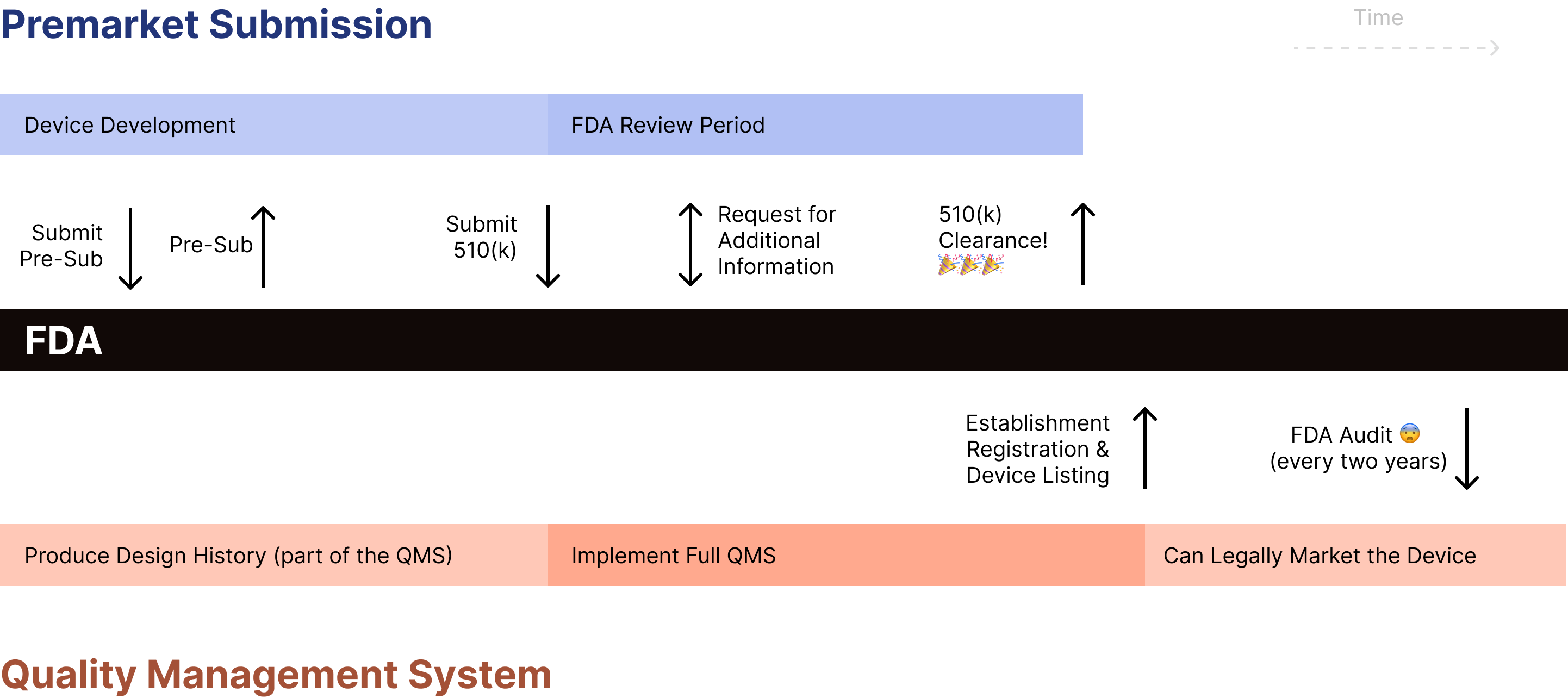
FDA Pre Subs: Best Practices FAQs and Examples

FDA 510(k) Submission: A Step By Step Guide On How To Prepare Yours
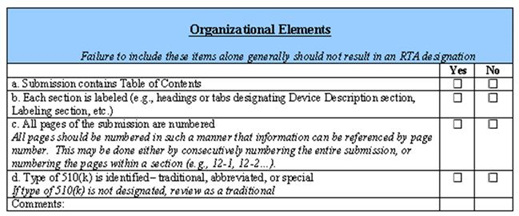
How To Make The Most Of Your Pre Submission Interactions With FDA

Pre FDA Audit Inspection Day Checklist Industrial Pharmacist

Ema Submission Timetable Ema Pre Authorization Guidelines HXYIMD

Regulatory Strategy for Pre IND Meetings with FDA: Why / regulatory

28 Minutes of Meeting Sample Templates
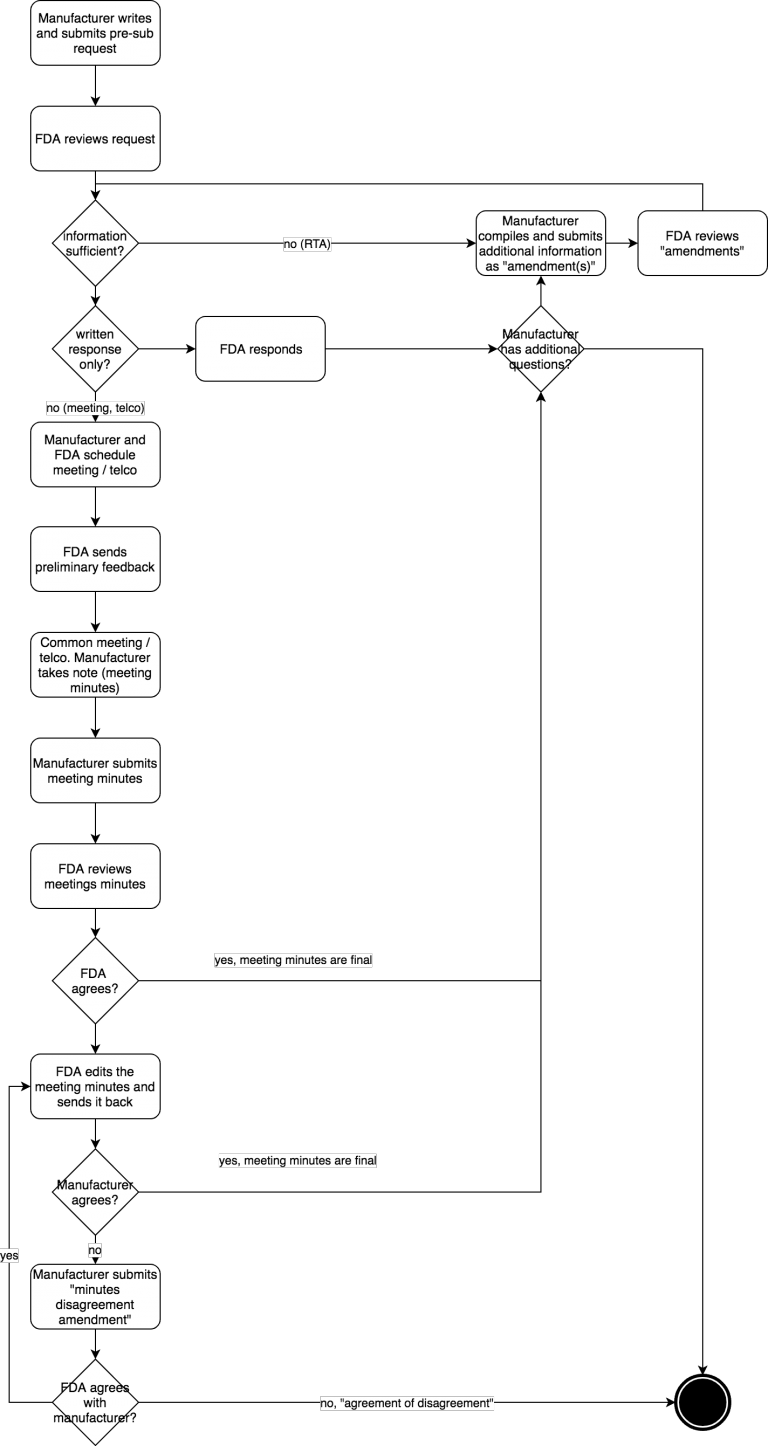
FDA Pre Submission Programm
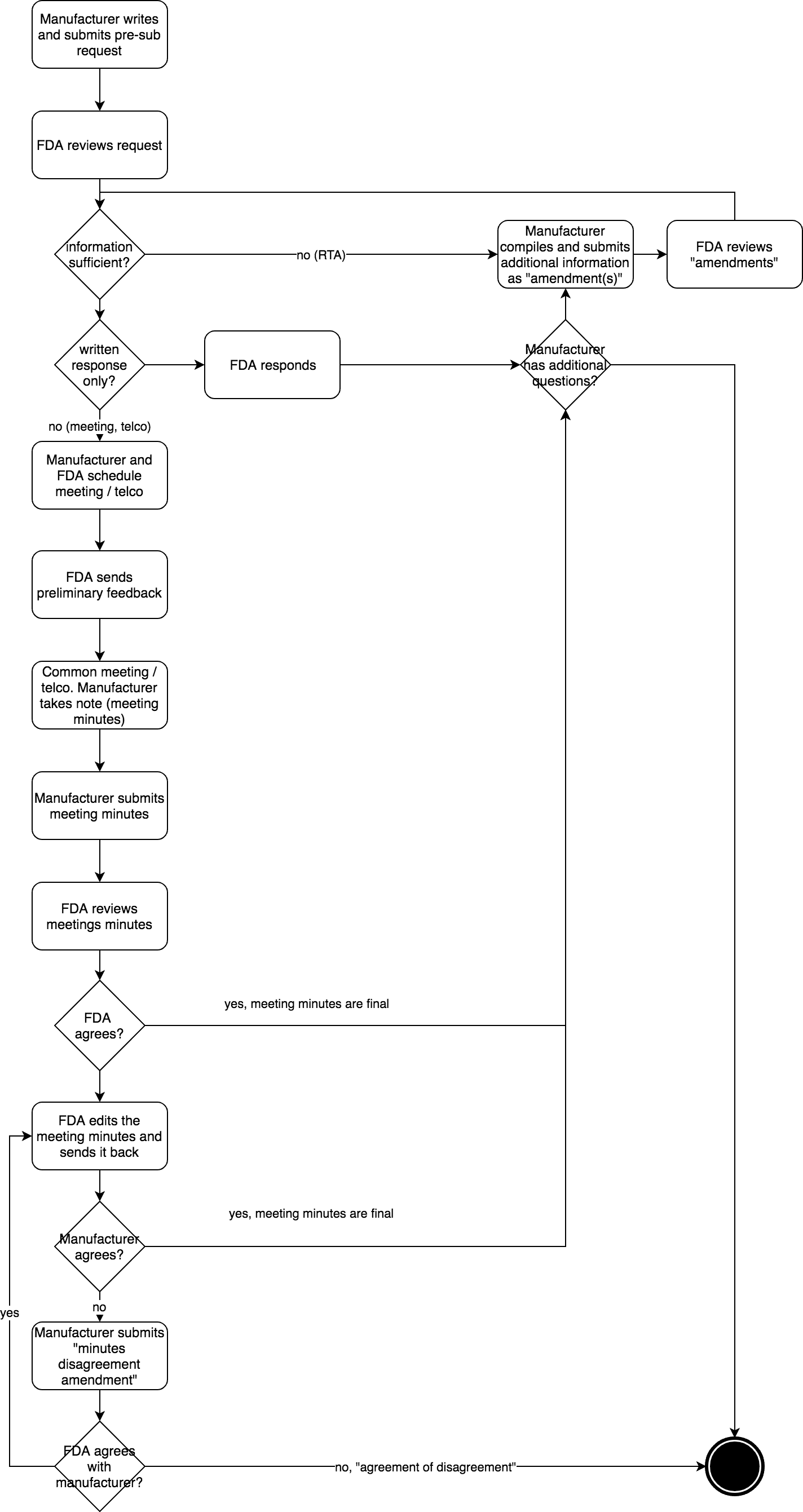
FDA Pre Submission Programm

Notification of a Manufacturing Discontinuance or Interruption: Does

Notification of a Manufacturing Discontinuance or Interruption: Does

Notification of a Manufacturing Discontinuance or Interruption: Does
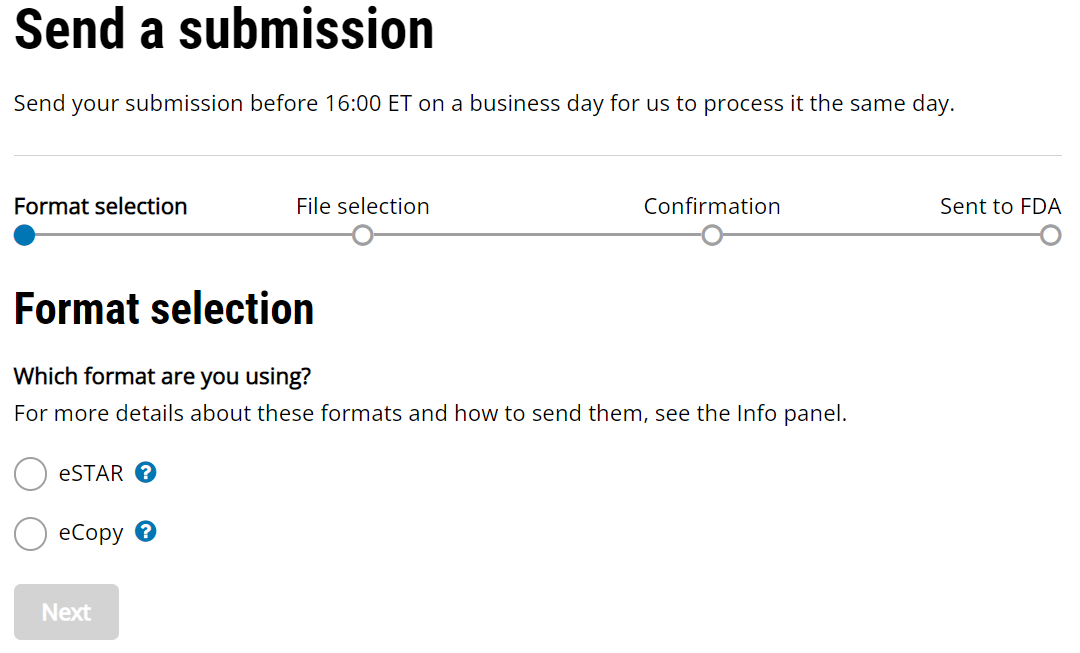
510k Electronic Submission Guidance for FDA 510k Submissions
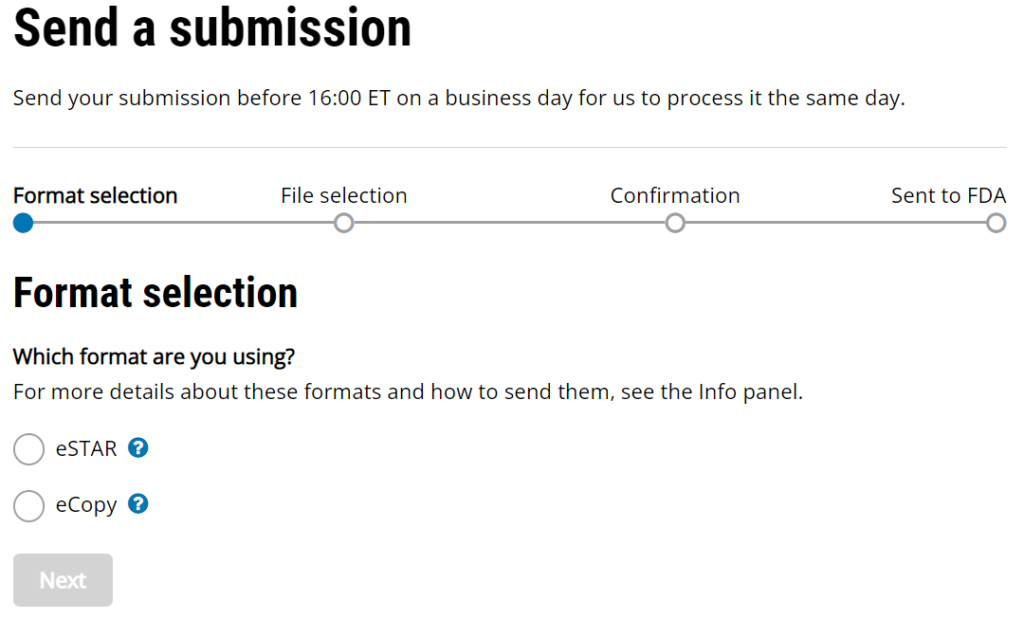
510k Electronic Submission Guidance for FDA 510k Submissions

What is the FDA eSTAR program?
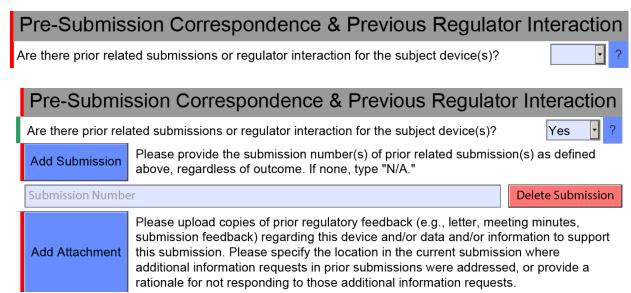
What is the FDA eSTAR program?
.jpg)
What is the FDA eSTAR program?
FREE 18 Submission Checklist Samples in MS Word Google Docs PDF

FREE 18 Submission Checklist Samples in MS Word Google Docs PDF
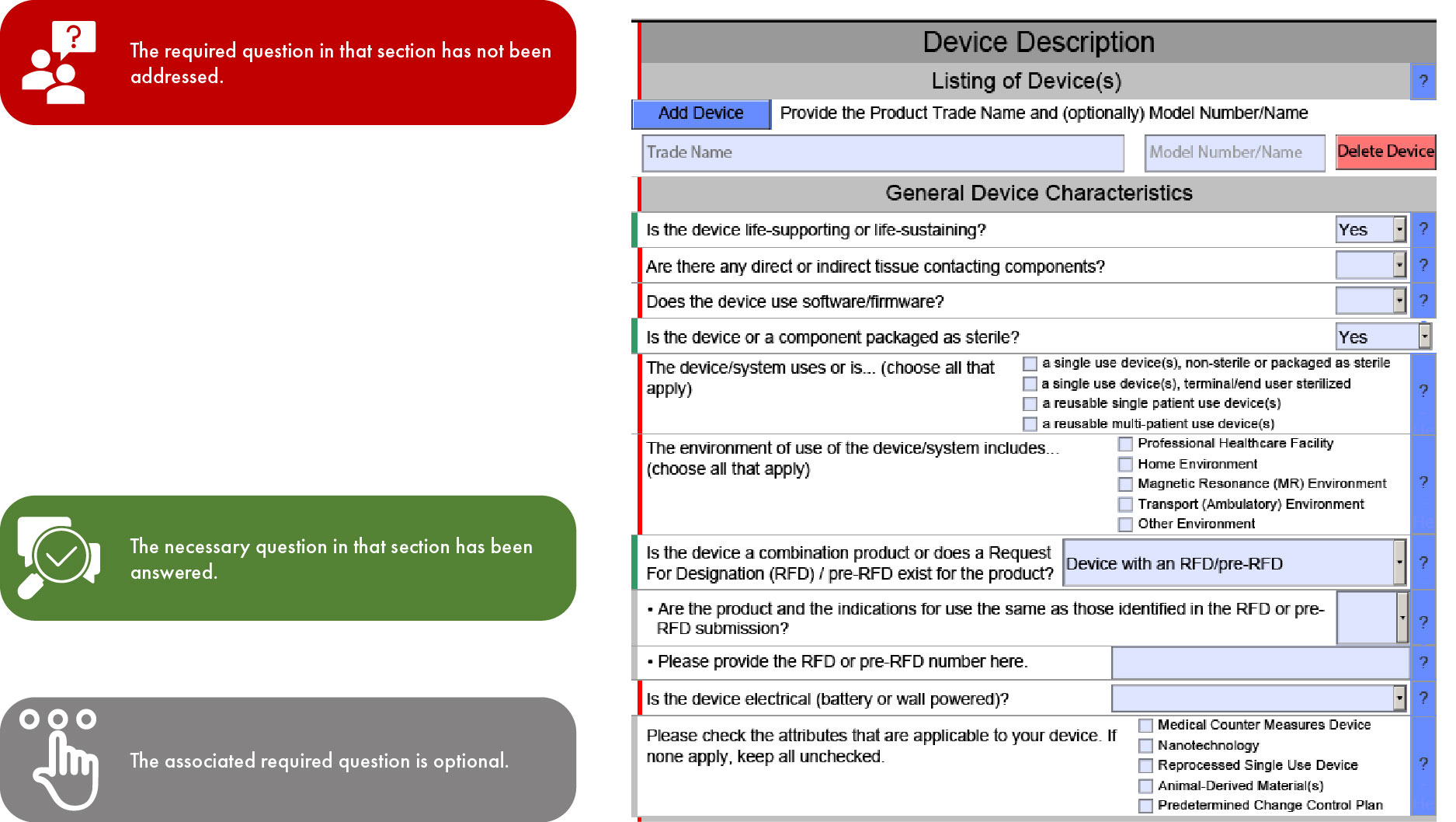
FDA eSTAR Program eSTAR Submissions 510(k) Submission
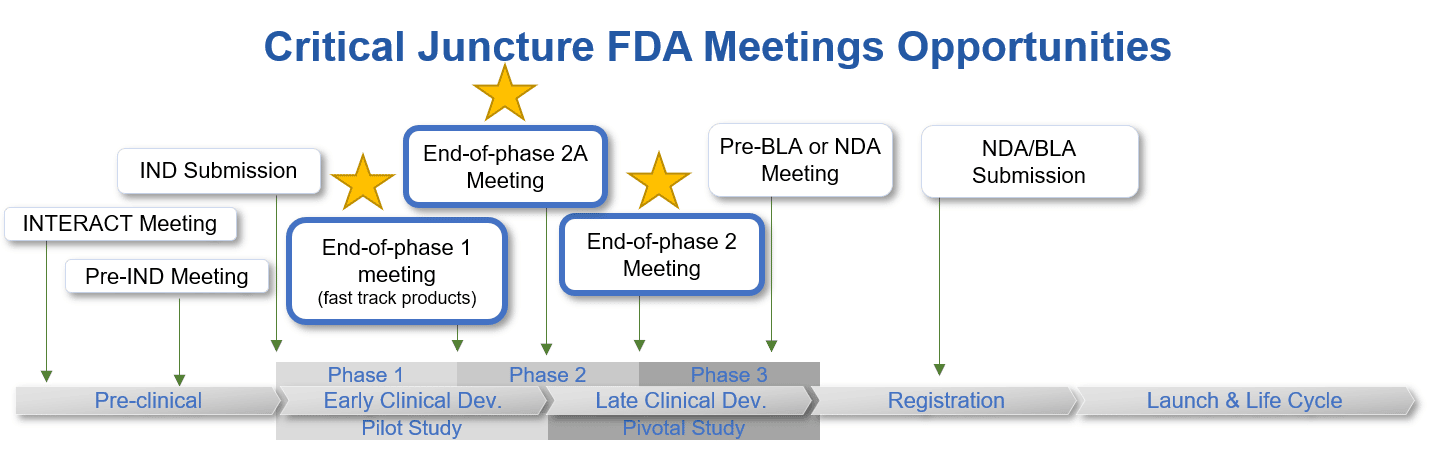
FDA Meeting Series: How When and What EOP Meetings

Medical Device Complaint Form Free Download

Assignment 2 6370 docx FDA Pre Submission Cover Letter Henry

Real 510(k) Example

How To Submit An Investigational New Drug (IND) Application

Virax Biolabs Partners with Emory University on ViraxImmune™ Clinical