Eu Mdr Certification Course
Here are some of the images for Eu Mdr Certification Course that we found in our website database.

EU MDR Certification Ad Tech Medical

Corsano Health Receives EU MDR Certification Corsano Health

EU MDR Certification Medical Device Regulation IAS

Norlase Receives EU MDR Certification

Vesalio announces EU MDR Certification Vesalio
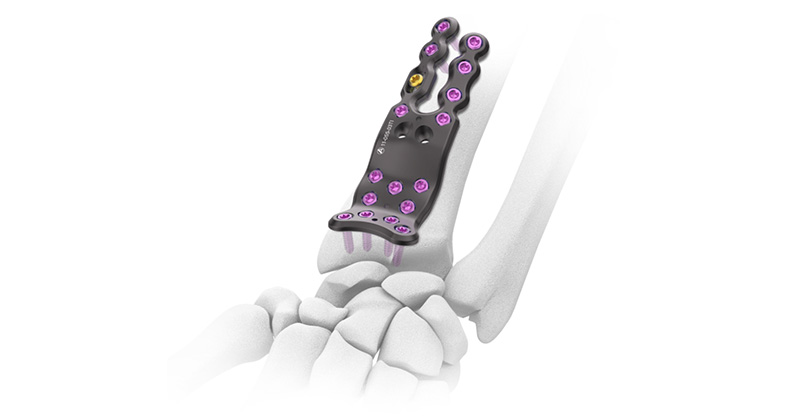
Auxein Achieves EU MDR Certification BONEZONE

EU MDR Certification granted

SunMed Earns EU MDR Certification AirLife

EU MDR Training Royed Training

EU MDR Training Royed Training

EU MDR Training Course Comprehensive Online Certification MDR

EU CE MDR certification
Eu Mdr Certification prntbl concejomunicipaldechinu gov co

Eu Mdr Certification prntbl concejomunicipaldechinu gov co

Eu Mdr Certification prntbl concejomunicipaldechinu gov co

Silhouette Soft achieves EU MDR Certification Sinclair United Kingdom
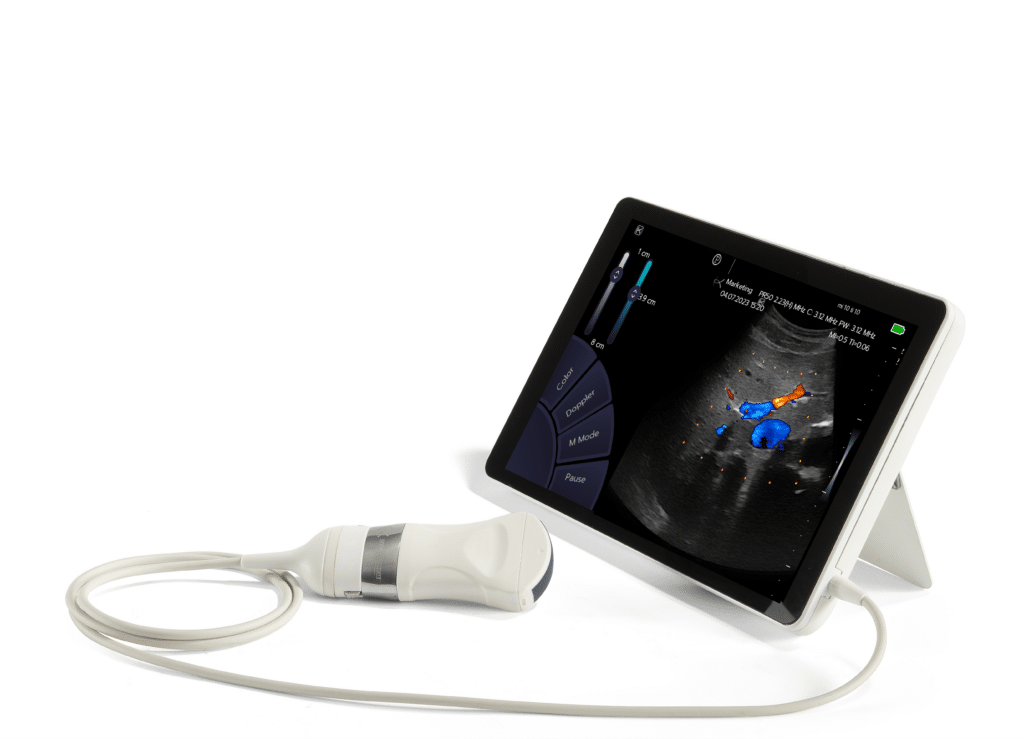
Sonoscanner EU MDR certified Sonoscanner

G H Orthodontics Achieves EU MDR Certification Business Wire

Qure ai Awarded EU MDR Certification for Its AI solutions AI Tech Park
Medical Device Regulation MDR 2017/745 Course and Certificate

Medical Device Regulation MDR 2017/745 Course and Certificate

Bonebridge Bonebridge Receives EU MDR Certification

The Magseed® Marker Achieves EU Medical Device Regulation Certification

Celluma Light Therapy Receives EU MDR Certification
EU MDR 2017/745 Training Course Oriel STAT A MATRIX

Celluma Light Therapy Receives EU MDR Certification Company expands pr

Success: Nipro receives EU MDR certification for medical devices Nipro

Summit Medical #39 s EU MDR Certification: Pioneering Quality and

Medikro obtains EU MDR certification reinforcing commitment to quality

Neauvia #39 sDermal Fillers Attain New EU MDR Certification

Medimaps secures EU MDR certification for its AI image processing

News Congratulations to Kangyuan Medical for obtaining EU MDR CE
LifeSignals Receives EU MDR Certification for UbiqVue™ 2A

News Congratulations to Kangyuan Medical for obtaining EU MDR CE

Eschmann achieves EU MDR certification for its autoclaves Eschmann
Mindray defibrillators amongst the world s first to receive EU MDR
Mindray defibrillators amongst the world s first to receive EU MDR

DentalMonitoring Sets New Standard in Orthodontic Remote Monitoring

Smart Blood Analytics Swiss Achieves EU MDR Certification for VIRUS vs
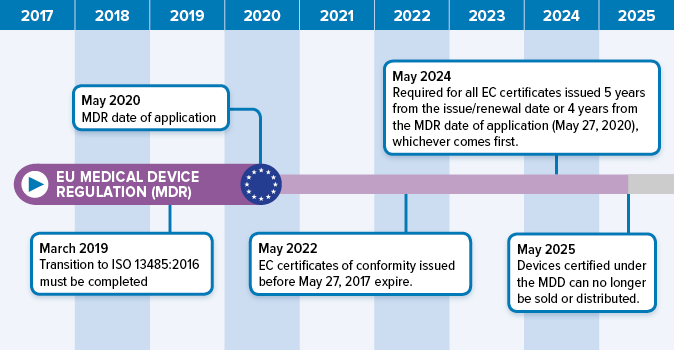
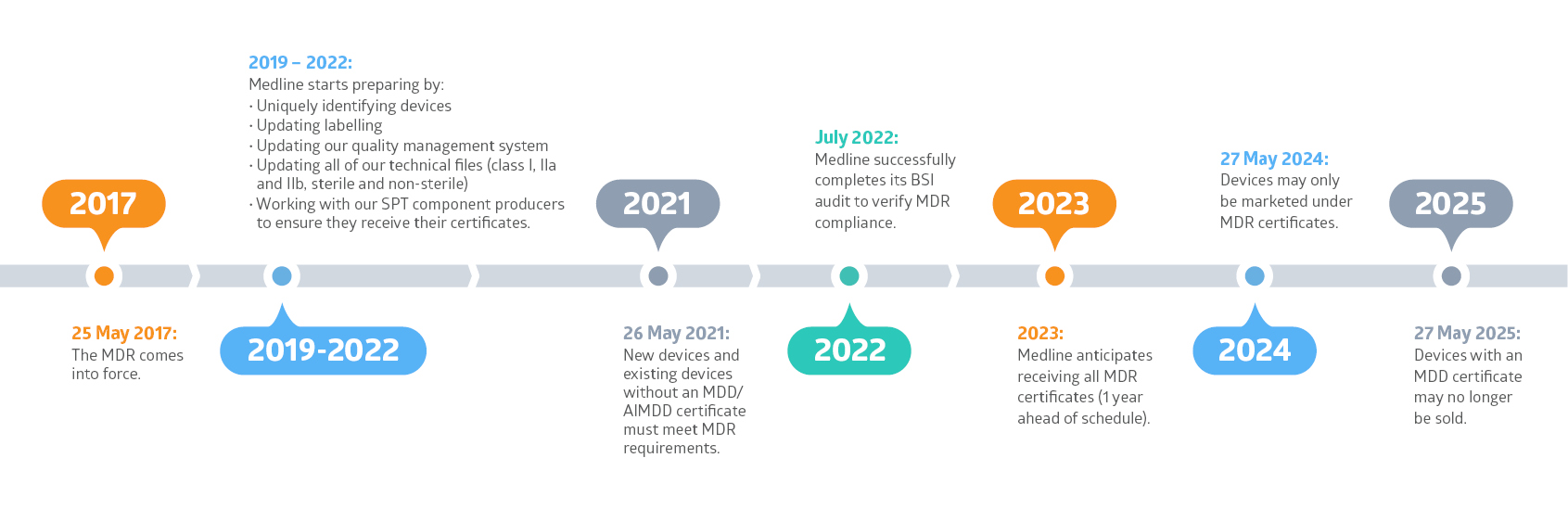
EU MDR Transition Timelines and Deadlines for 2017/745

Smart Blood Analytics Swiss Achieves EU MDR Certification for VIRUS vs

A Game Changer in Antimicrobial Resistance Battle: VIRUS vs BACTERIA

Infinium Medical announces EU MDR Certification for the OMNI Series and

Traceability Requirements For Medical Devices in EU MDR Operon Strategist

What does it mean that your AI Clinical Decision Support Software has

Bharat Rubber Works becomes one of the first Asian companies to receive
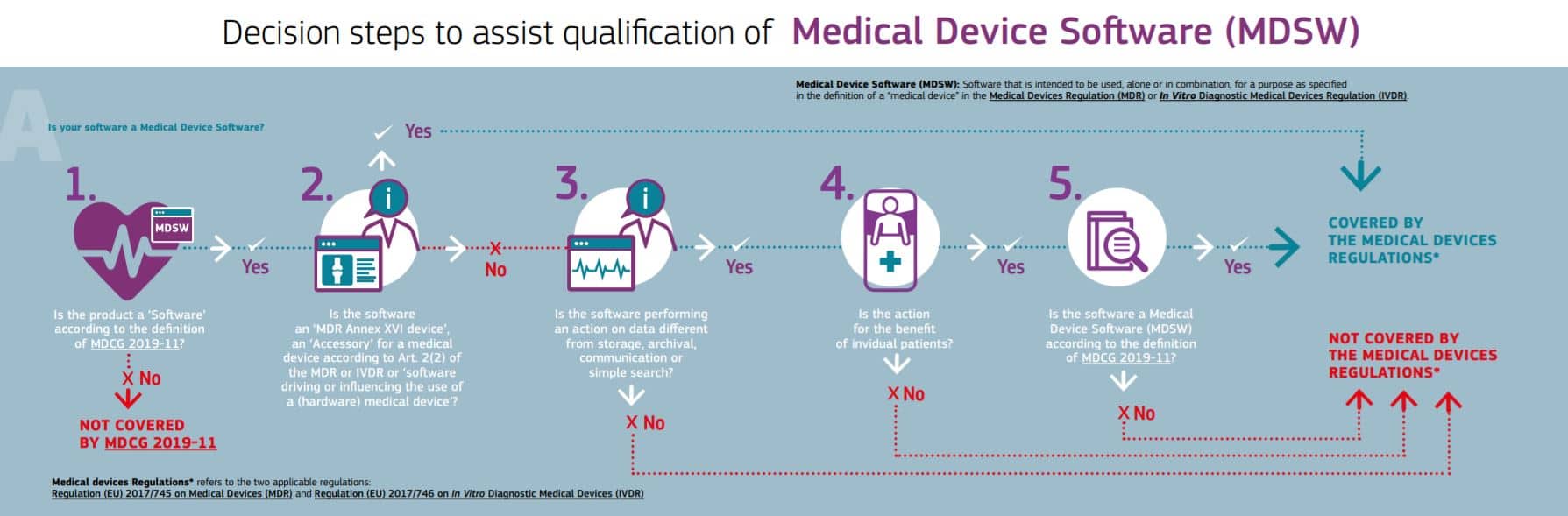
EU MDR EU Medical Device Regulation IVDR compliance

DentalMonitoring Sets New Standard in Orthodontic Remote Monitoring

Butterfly Network Receives EU MDR Certification for Butterfly iQ
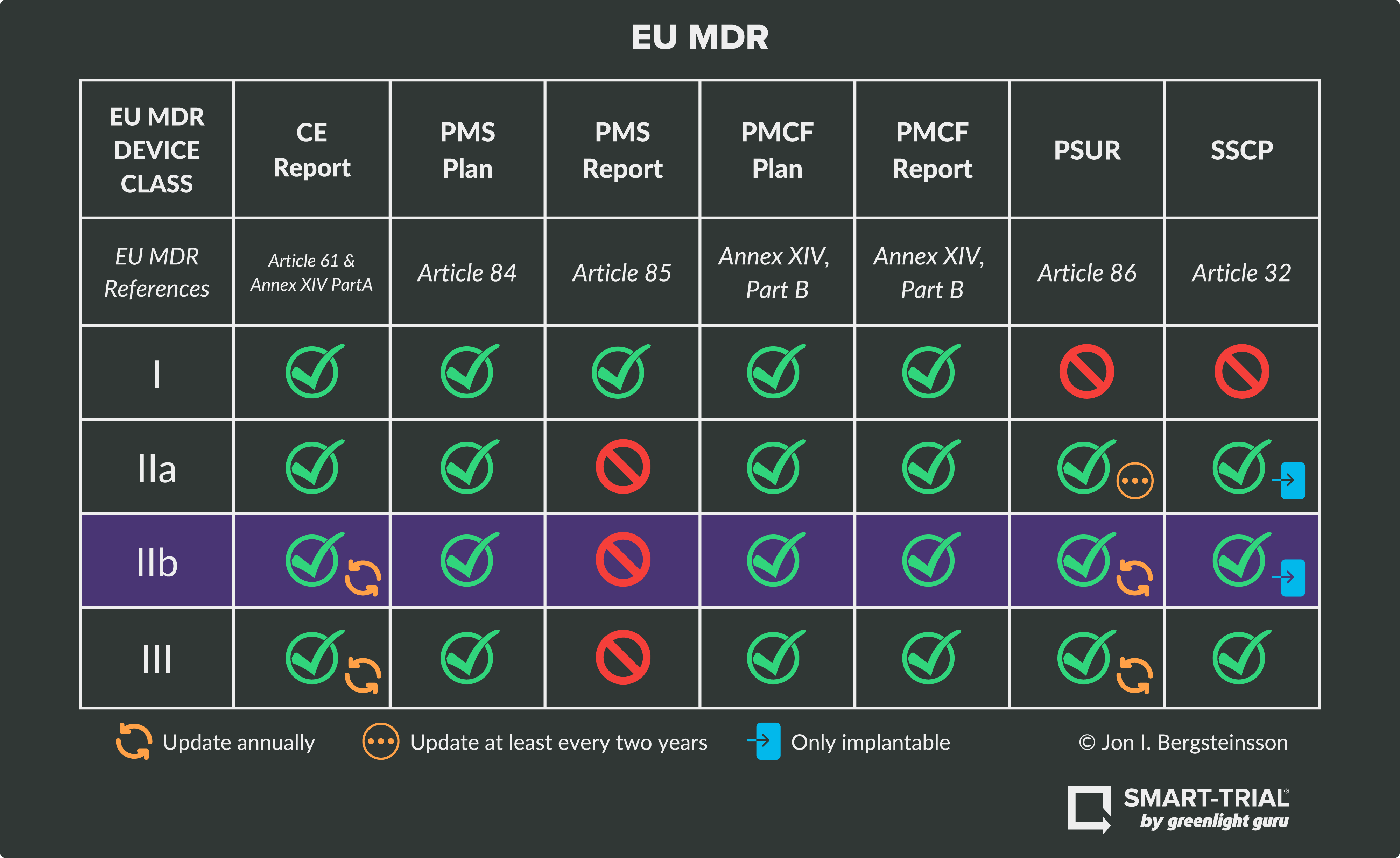
Ultimate Guide to Device Class Requirements under EU MDR

Free Mini Course EU MDR 2017/745 (Medical Device regulation training)
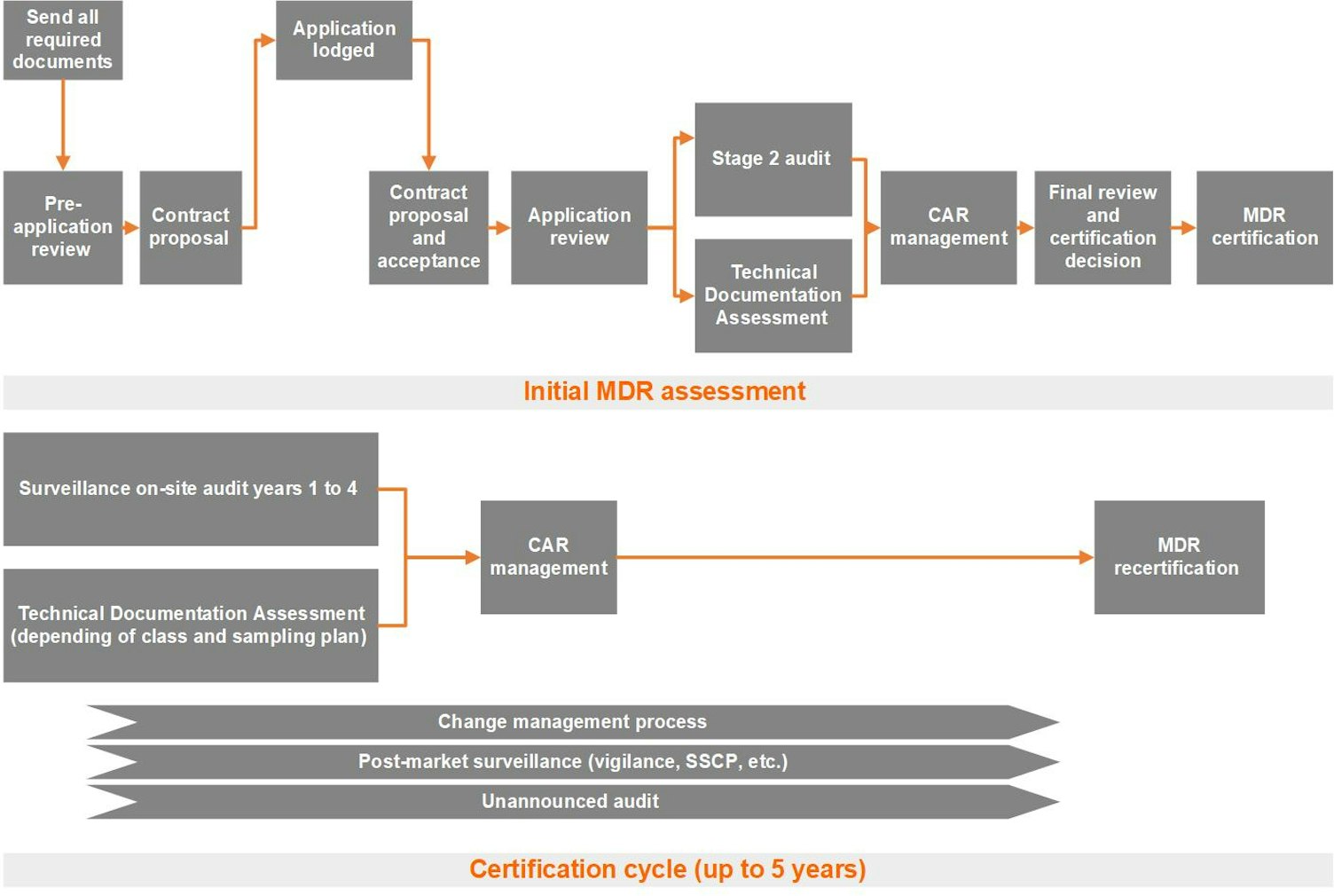
The Medical Device Regulation (MDR) (EU) 2017/745 Certification Process
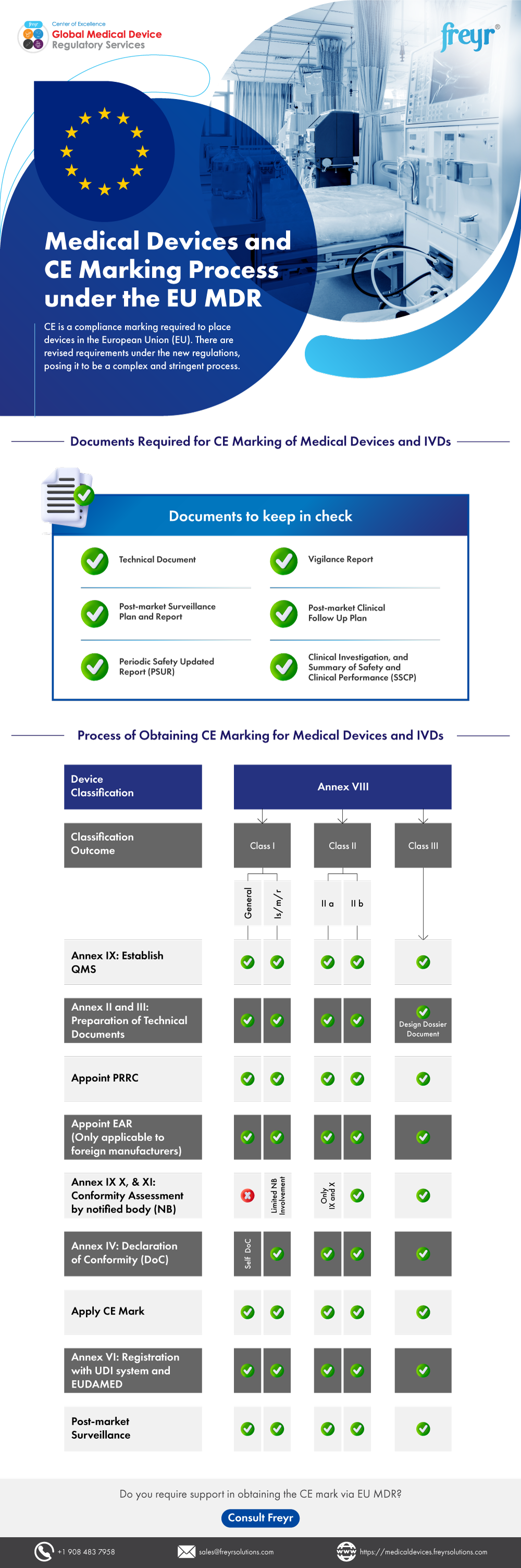
Medical Devices and CE Marking Process under the EU MDR Freyr

LifeSignals UbiqVue 2A biosensor gains EU certification NotebookCheck

MDR Requirements for Reusable Surgical Instruments

Courses Easy Medical Device School

Build a winning strategy for EU MDR Compliance Free course Comidoc

CE Kennzeichnung für Medizinprodukte in der EU gemäß MDR 2017/745

EU MDR: EU Medical Device Regulations Chapter 10: Final Provisions