Eu Mdr Certification
Here are some of the images for Eu Mdr Certification that we found in our website database.

EU MDR certification

EU MDR Certification Ad Tech Medical

Corsano Health Receives EU MDR Certification Corsano Health

EU MDR Certification Medical Device Regulation IAS

Norlase Receives EU MDR Certification

Vesalio announces EU MDR Certification Vesalio

X Guide Receives EU MDR Certification

EU MDR Certification granted
Auxein Achieves EU MDR Certification BONEZONE

SunMed Earns EU MDR Certification AirLife
Addressing the EU MDR and IVDR Certification Bottleneck Blog AssurX

EU CE MDR certification
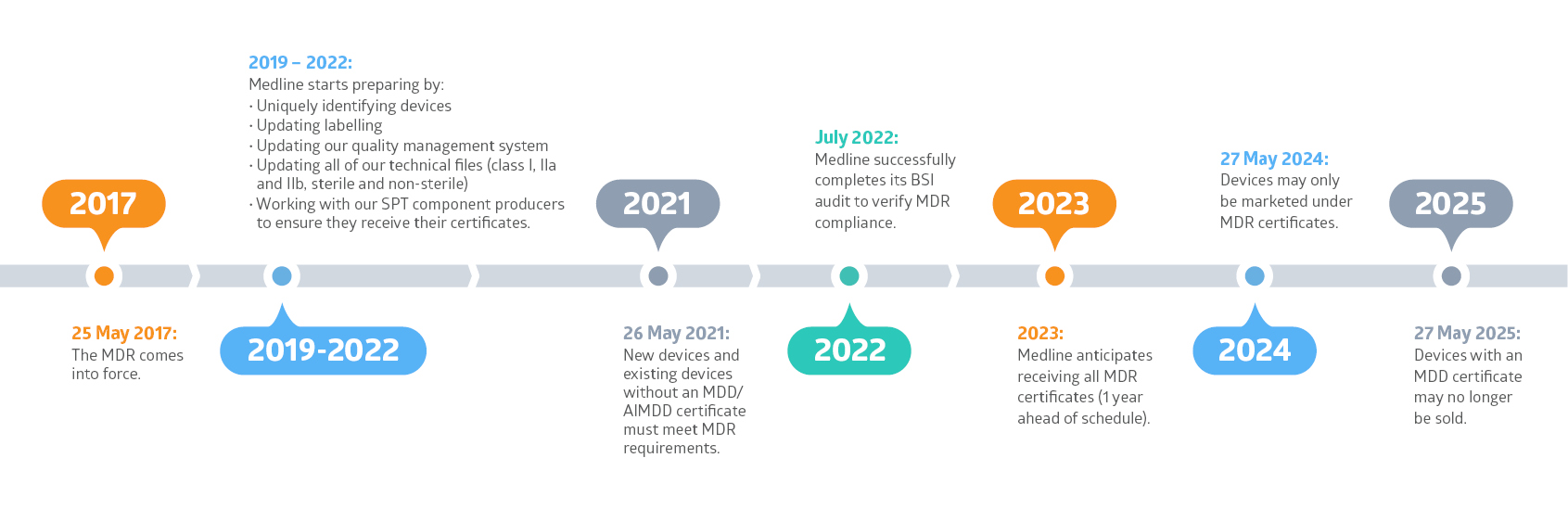
Eu Mdr Certification prntbl concejomunicipaldechinu gov co

Eu Mdr Certification prntbl concejomunicipaldechinu gov co

Eu Mdr Certification prntbl concejomunicipaldechinu gov co

Eu Mdr Certification prntbl concejomunicipaldechinu gov co
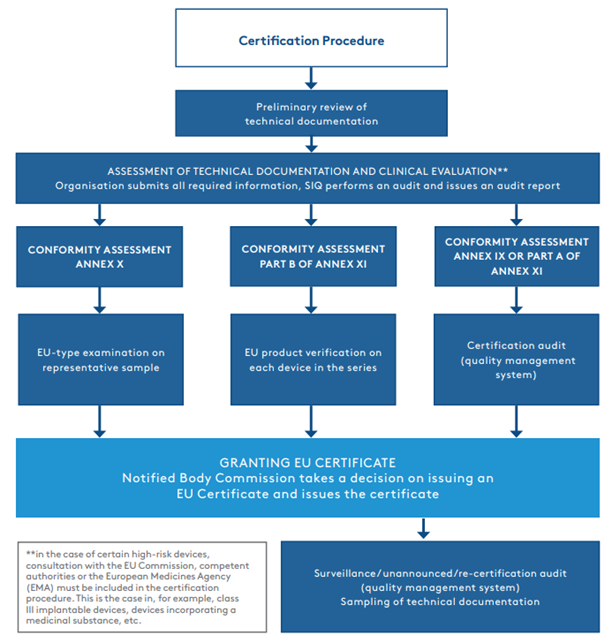
Eu Mdr Certification prntbl concejomunicipaldechinu gov co
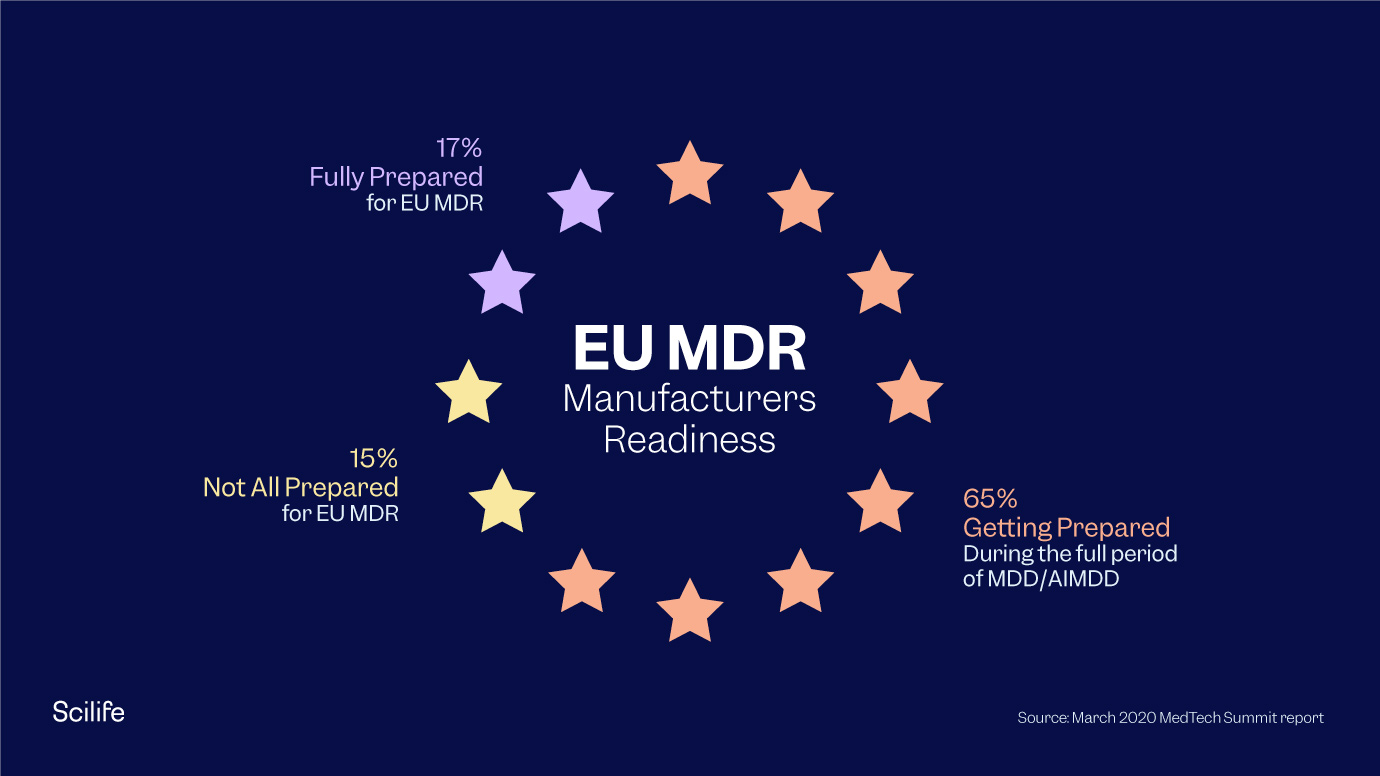
Eu Mdr Certification prntbl concejomunicipaldechinu gov co

Eu Mdr Certification prntbl concejomunicipaldechinu gov co

Eu Mdr Certification prntbl concejomunicipaldechinu gov co

Eu Mdr Certification prntbl concejomunicipaldechinu gov co

Eu Mdr Certification prntbl concejomunicipaldechinu gov co
Sonoscanner EU MDR certified Sonoscanner

G H Orthodontics Achieves EU MDR Certification Business Wire

Qure ai Awarded EU MDR Certification for Its AI solutions AI Tech Park

Bonebridge Bonebridge Receives EU MDR Certification

The Magseed® Marker Achieves EU Medical Device Regulation Certification

MedSafety Solutions Earns EU MDR Certification
Celluma Light Therapy Receives EU MDR Certification

Celluma Light Therapy Receives EU MDR Certification

Celluma Light Therapy Receives EU MDR Certification Company expands pr

Success: Nipro receives EU MDR certification for medical devices Nipro

Paxman Scalp Cooling System receives EU MDR certification Paxman

Summit Medical #39 s EU MDR Certification: Pioneering Quality and
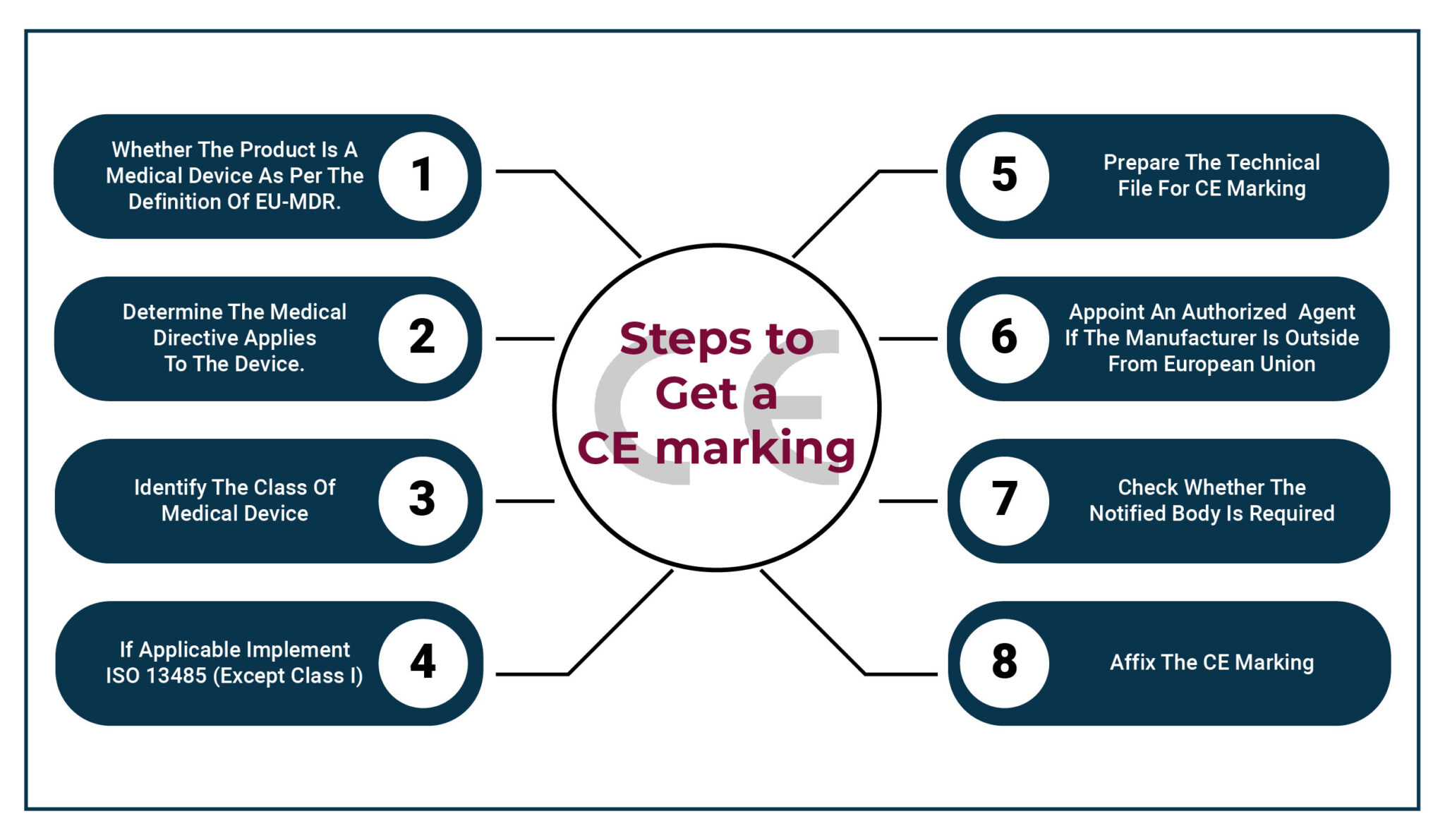
EU MDR Affected CE Marking: Complete Guide for Medical Device

Medtrade receives EU MDR Certification for the Celox CE Range
Platform24 s triaging software achieves EU MDR certification Platform24

Neauvia #39 sDermal Fillers Attain New EU MDR Certification

Medimaps secures EU MDR certification for its AI image processing

News Congratulations to Kangyuan Medical for obtaining EU MDR CE
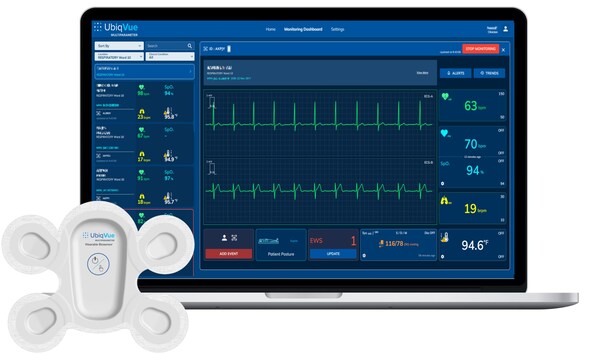
LifeSignals Receives EU MDR Certification for UbiqVue™ 2A

How MDR Class IIa Certification Impacts Digital Health Platforms in the EU

Mindray defibrillators amongst the world s first to receive EU MDR

Eschmann achieves EU MDR certification for its autoclaves Eschmann
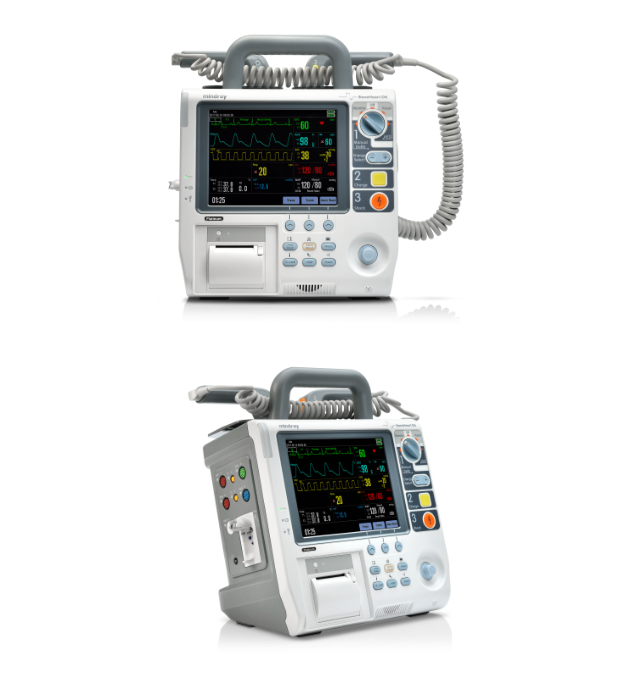
Mindray defibrillators amongst the world s first to receive EU MDR
Mindray defibrillators amongst the world s first to receive EU MDR

Orthodontic News DentalMonitoring Sets New Standard in Orthodontic

DentalMonitoring Sets New Standard in Orthodontic Remote Monitoring

Smart Blood Analytics Swiss Achieves EU MDR Certification for VIRUS vs

Smart Blood Analytics Swiss Achieves EU MDR Certification for VIRUS vs

A Game Changer in Antimicrobial Resistance Battle: VIRUS vs BACTERIA

Infinium Medical announces EU MDR Certification for the OMNI Series and

Bharat Rubber Works becomes one of the first Asian companies to receive

Avicenna AI secures EU MDR certification for five AI tools
Pearl Receives Industry First EU MDR Certification for Second Opinion

Butterfly Network Receives EU MDR Certification for Butterfly iQ
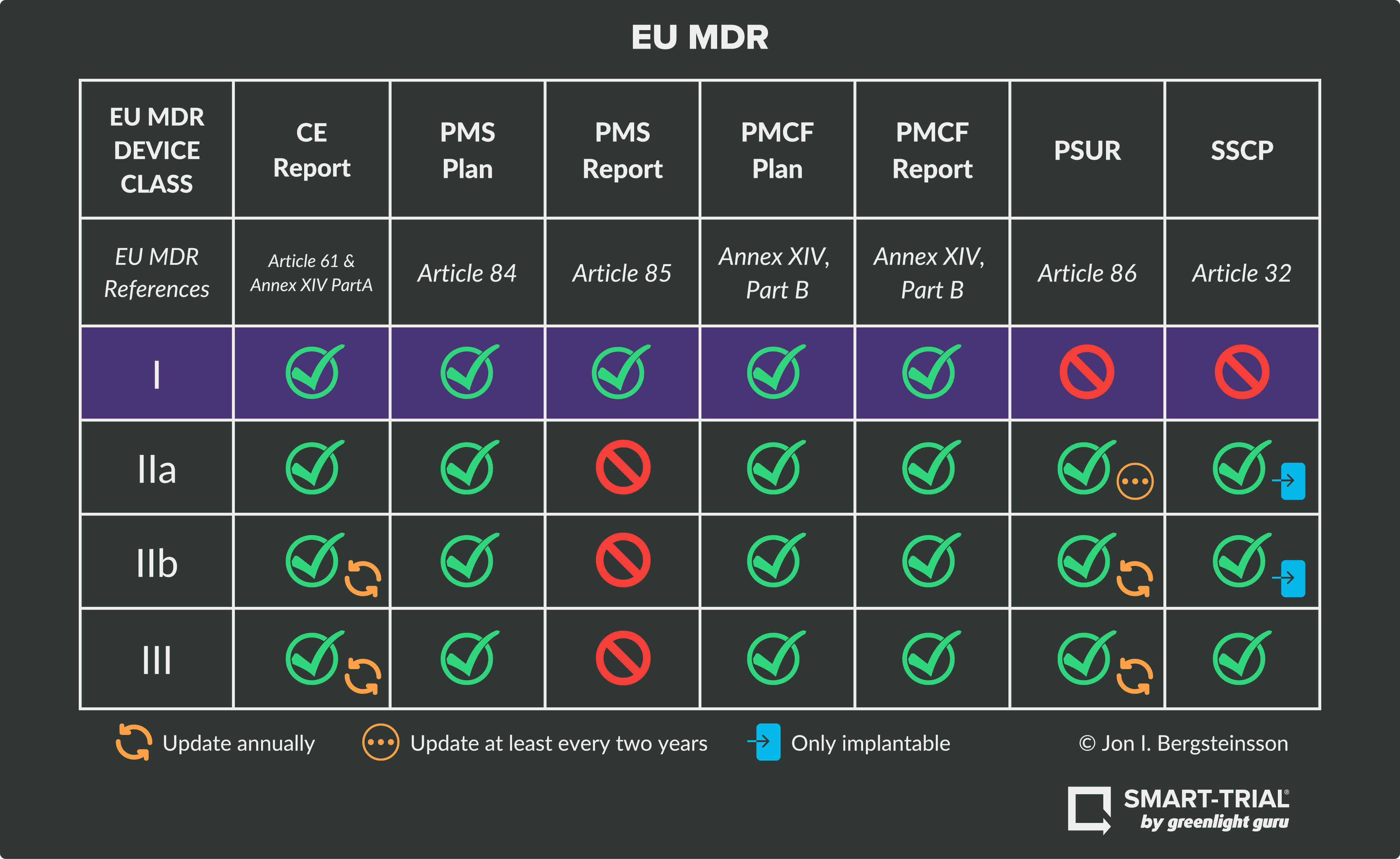
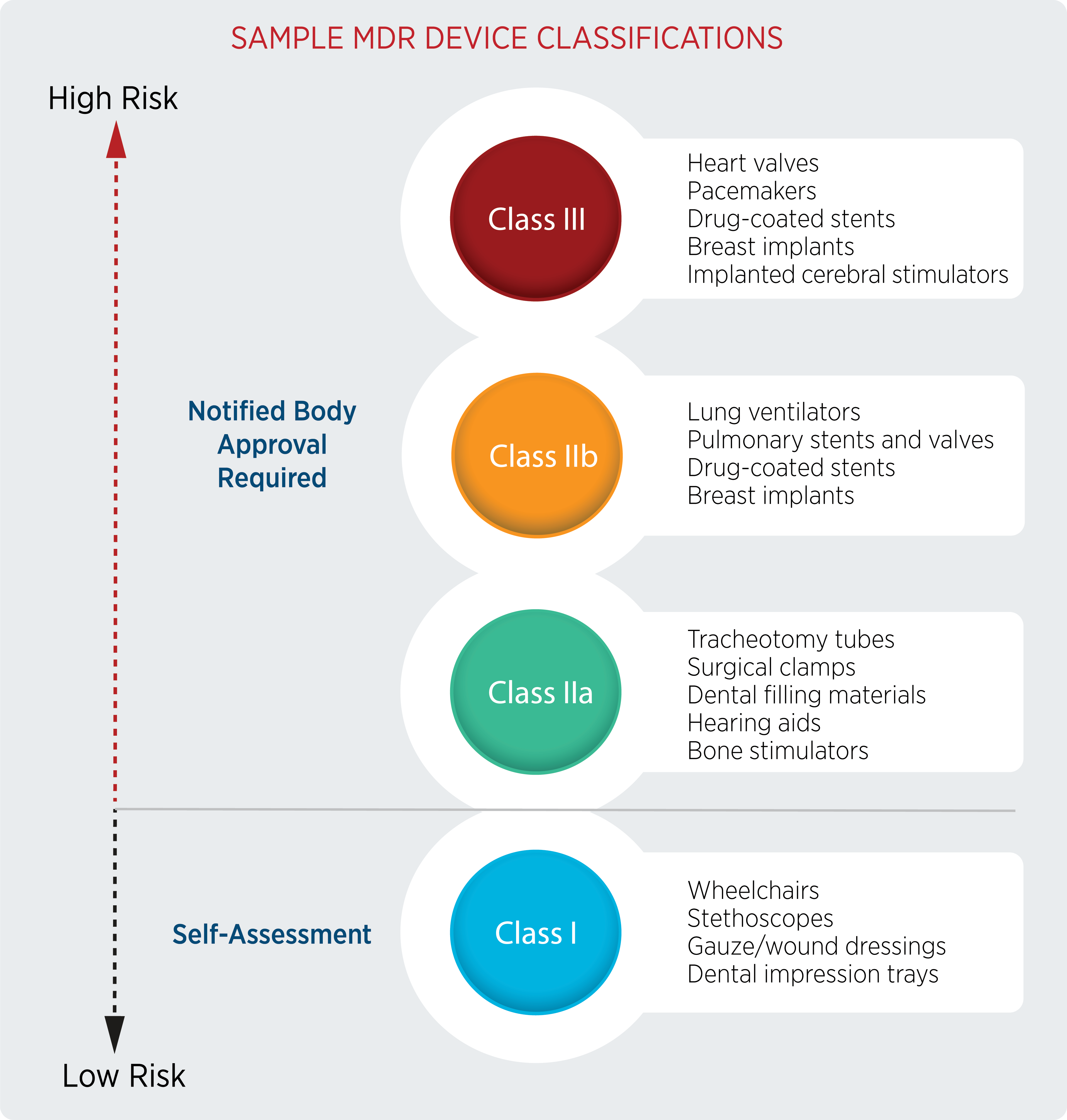
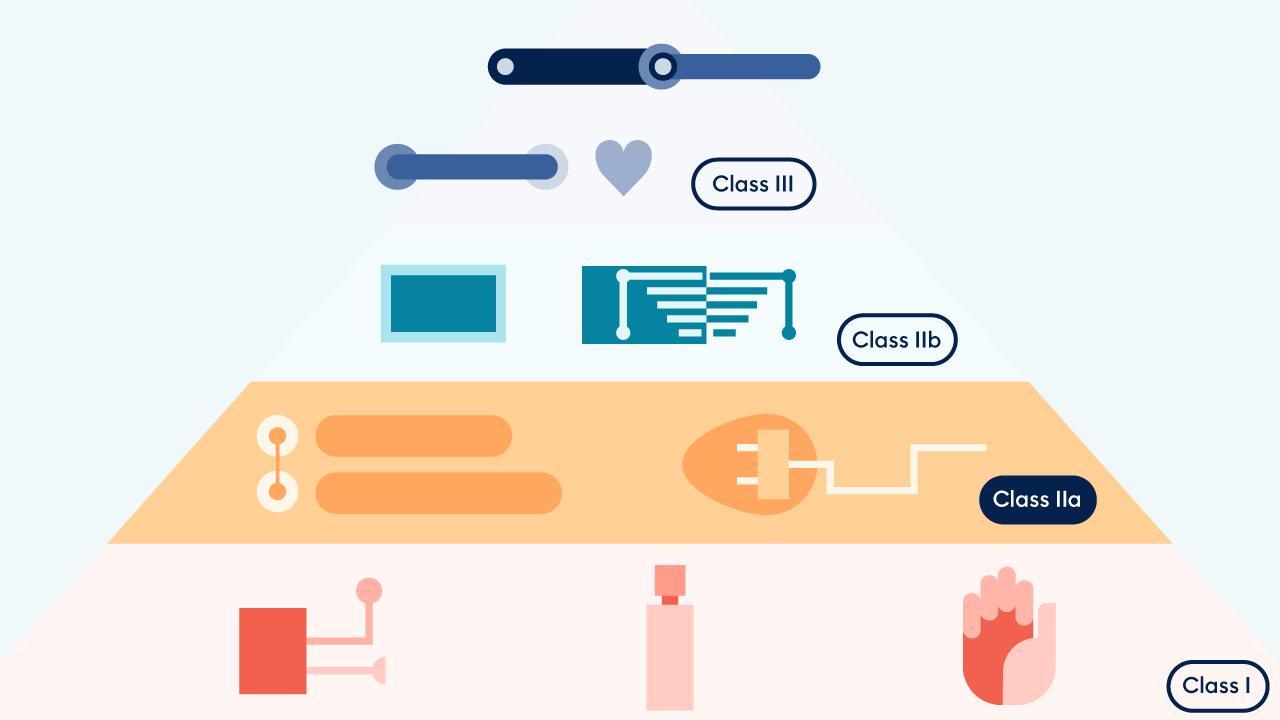
Ultimate Guide to Device Class Requirements under EU MDR

Auxein Strengthens Its Leadership in Global Orthopaedic Solutions with