Device History Record Template
Here are some of the images for Device History Record Template that we found in our website database.

SOP M 002 Device History Record Rev A Download Free PDF Quality

Device History Record Template

Device History Record Template
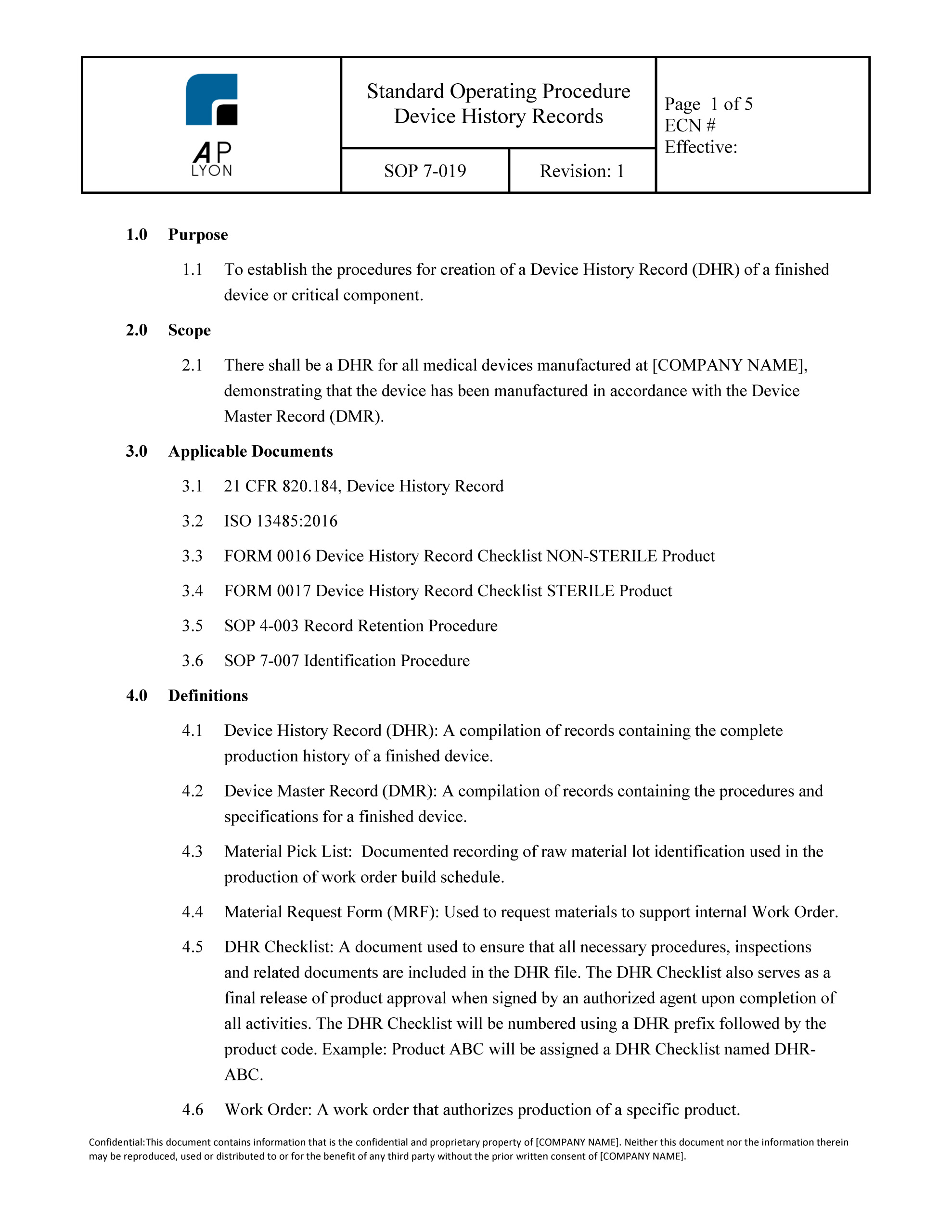
Device History Record Template

Device History Record Template
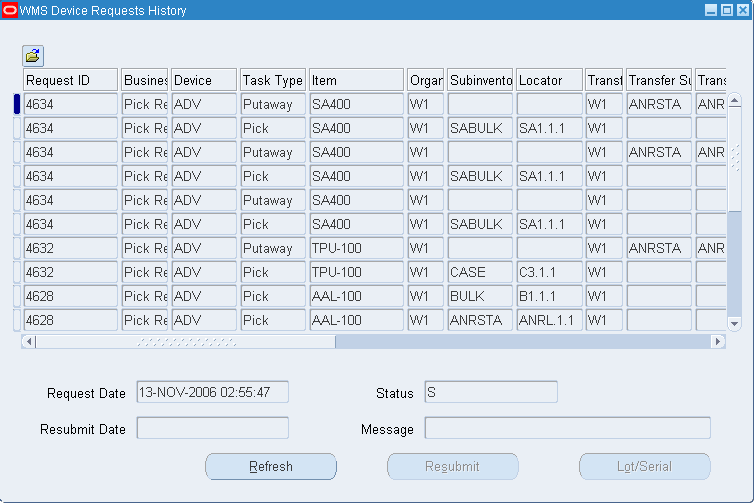
Device History Record Template

Device History Record Template
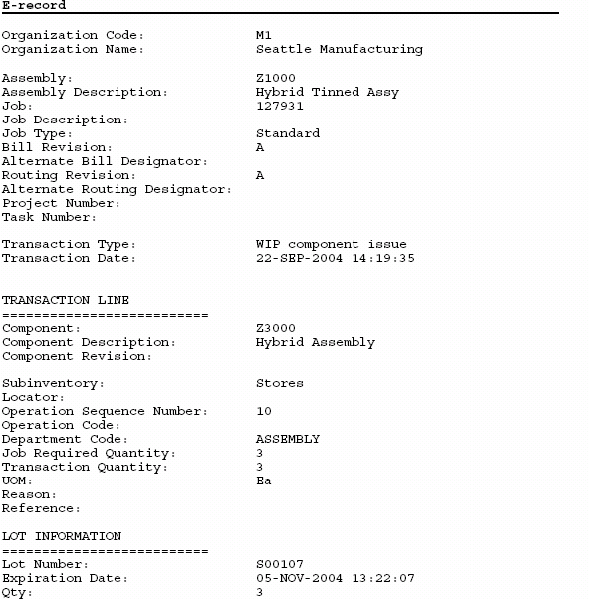
Device History Record Template
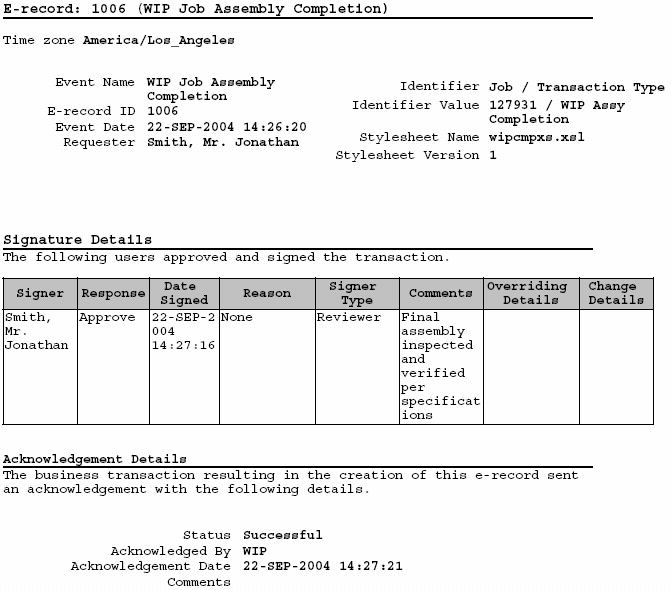
Device History Record Template
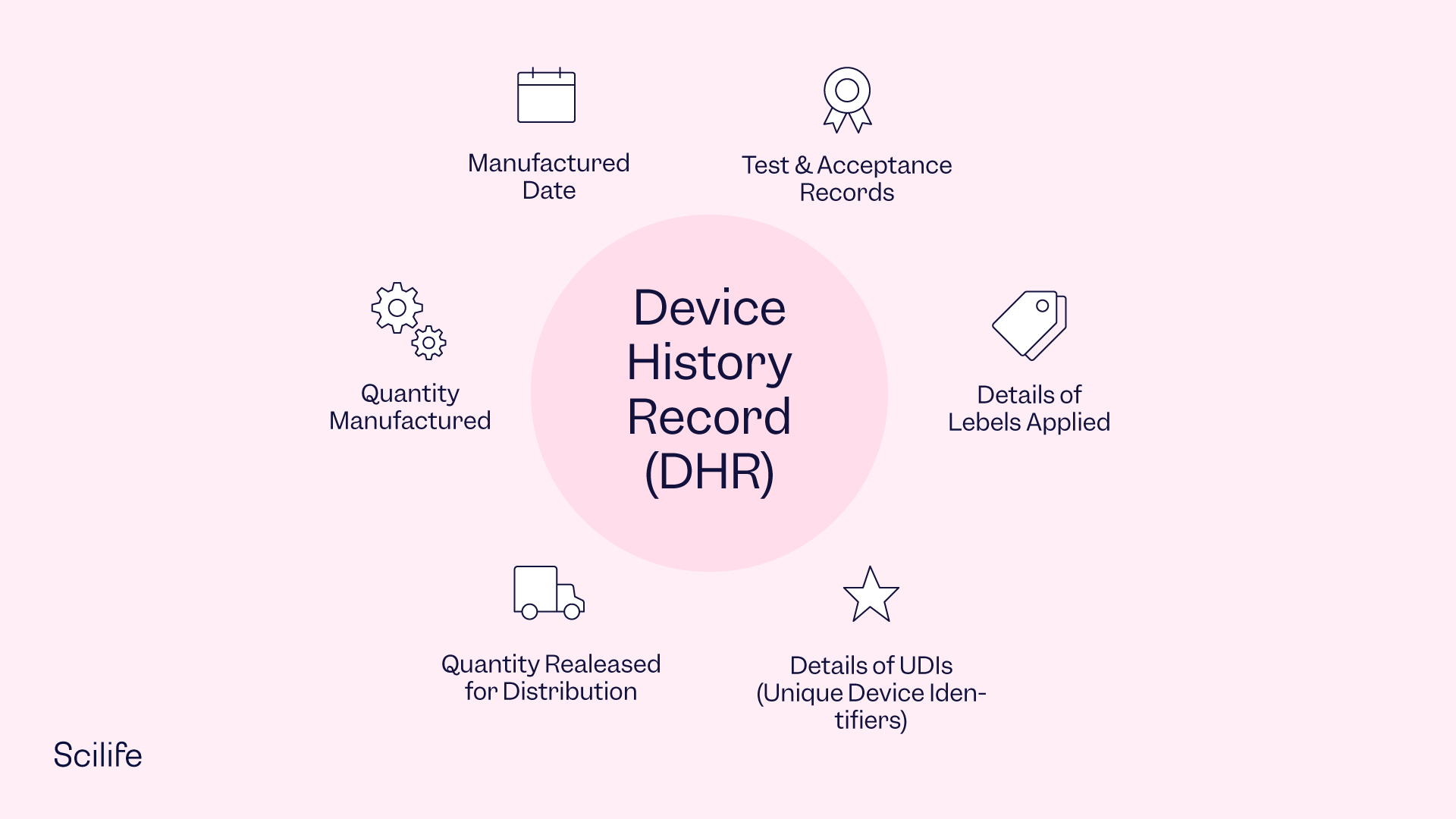
Device History Record Template

Device History Record Template
Device History Record Template

Device History Record

Device History Record (DHR): Definition Requirements and What It Includes

Device History Record (DHR): Definition Requirements and What It Includes

What Is a Device History Record (DHR) and Why Is It Important?
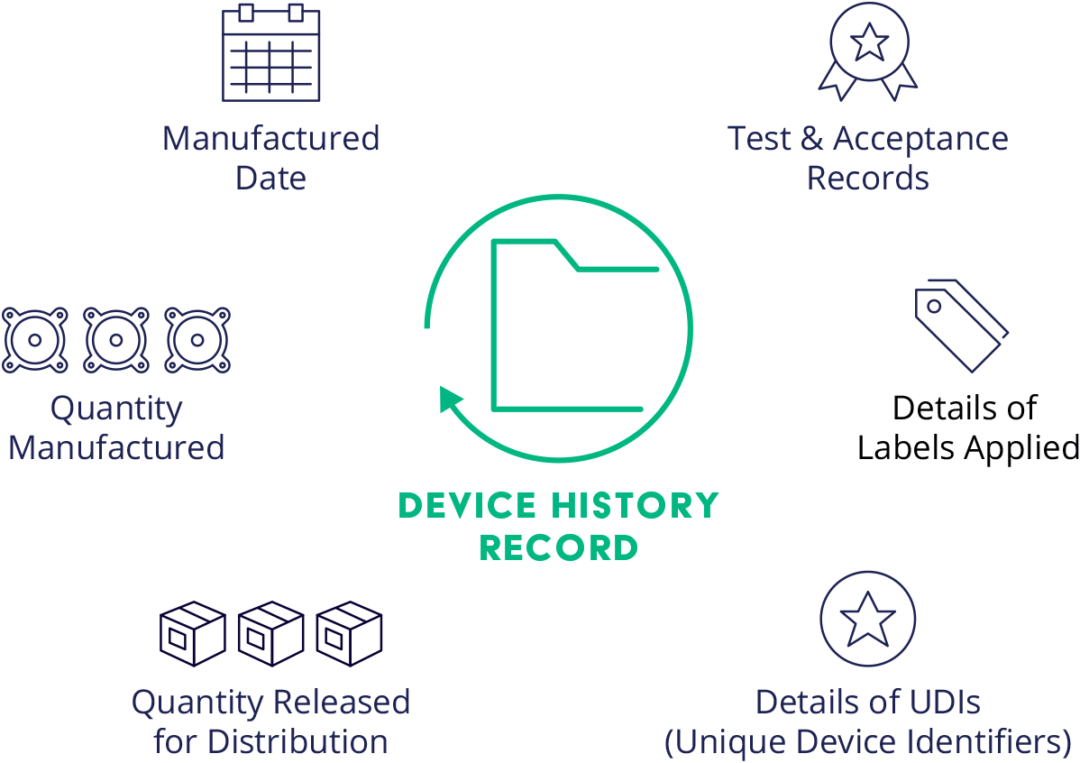
Device History Record (DHR) Definition Arena

Device Master Record Template
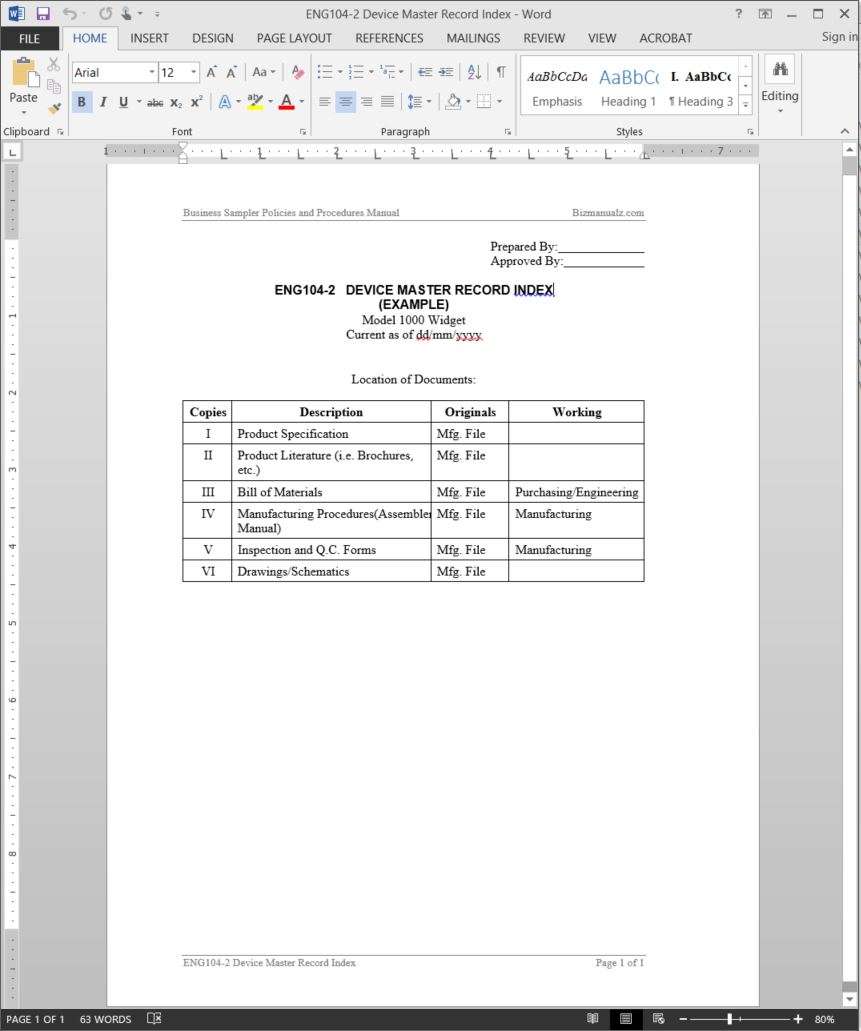
Device Master Record Template

Device History Record Procedure

Device Master Record Template

Device Master Record Template

Device History Record Procedure

(DHF) Device Master Record (DMR) Device History

Design History File (DHF) vs Device Master Record (DMR) vs Device
Medical Device Design History File Template

Electronic Device History Record (eDHR): What You Should Know

Electronic Device History Record (eDHR): What You Should Know

Batch Record Review Checklist Template/Example GMPDocs com

What Is Device Master Record (DMR) and Why Is It Important?

Medical Device Design History File Template prntbl
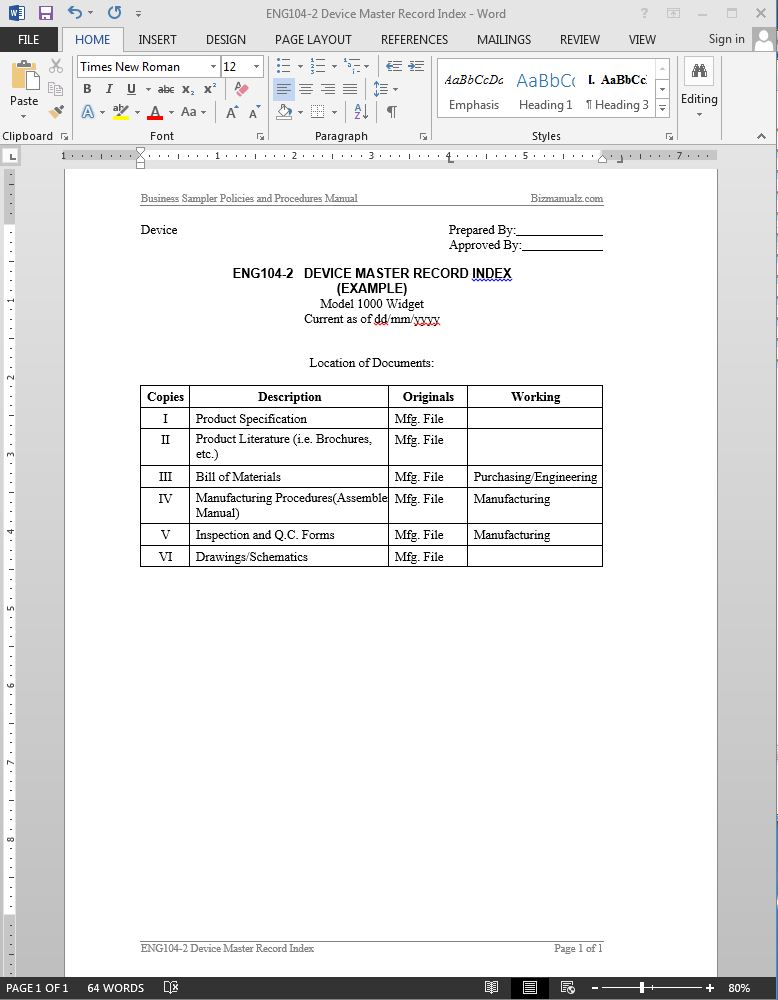
Device Master Record Index Template Word

Personal Medical History Template Employee Onboarding Template

Device History Record Template Fill Online Printable Fillable
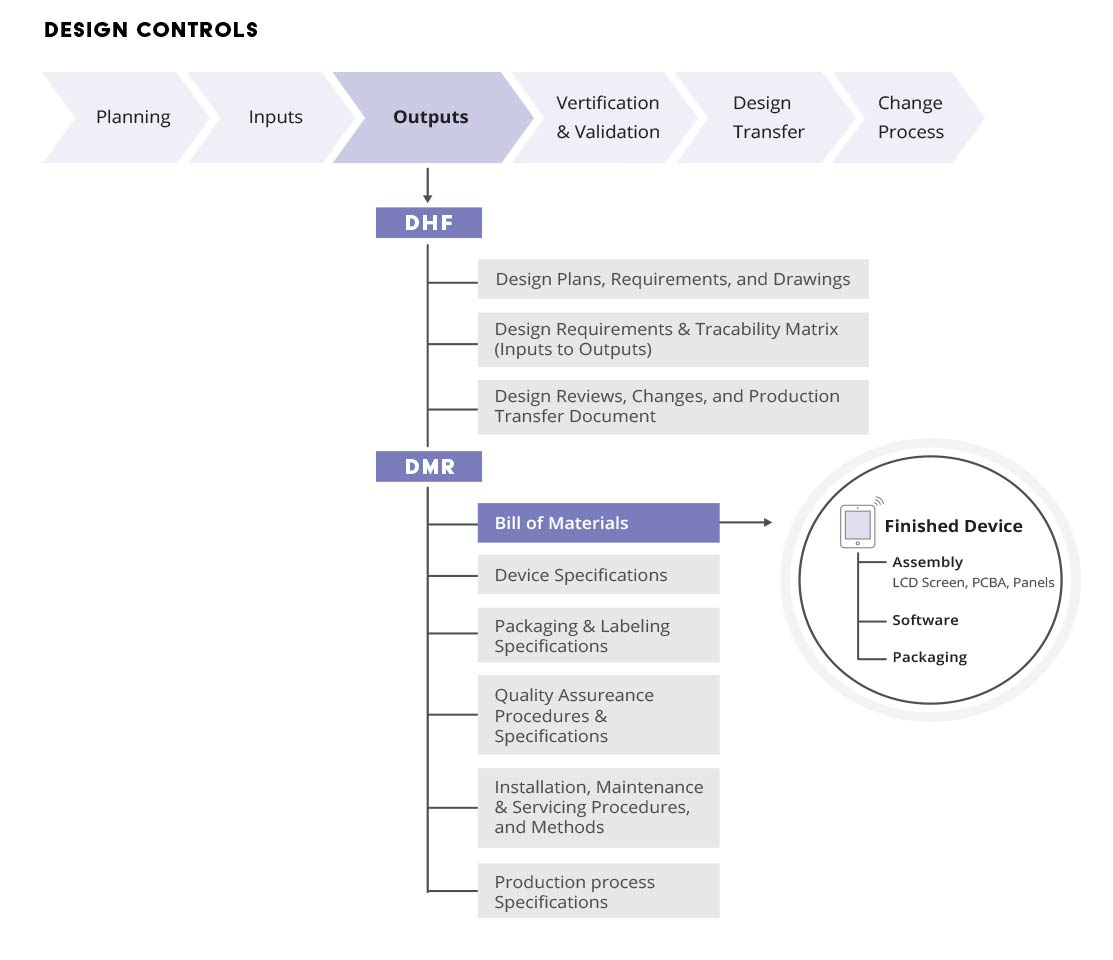
Managing The Device Master Record (DMR) Arena
Device Master Record
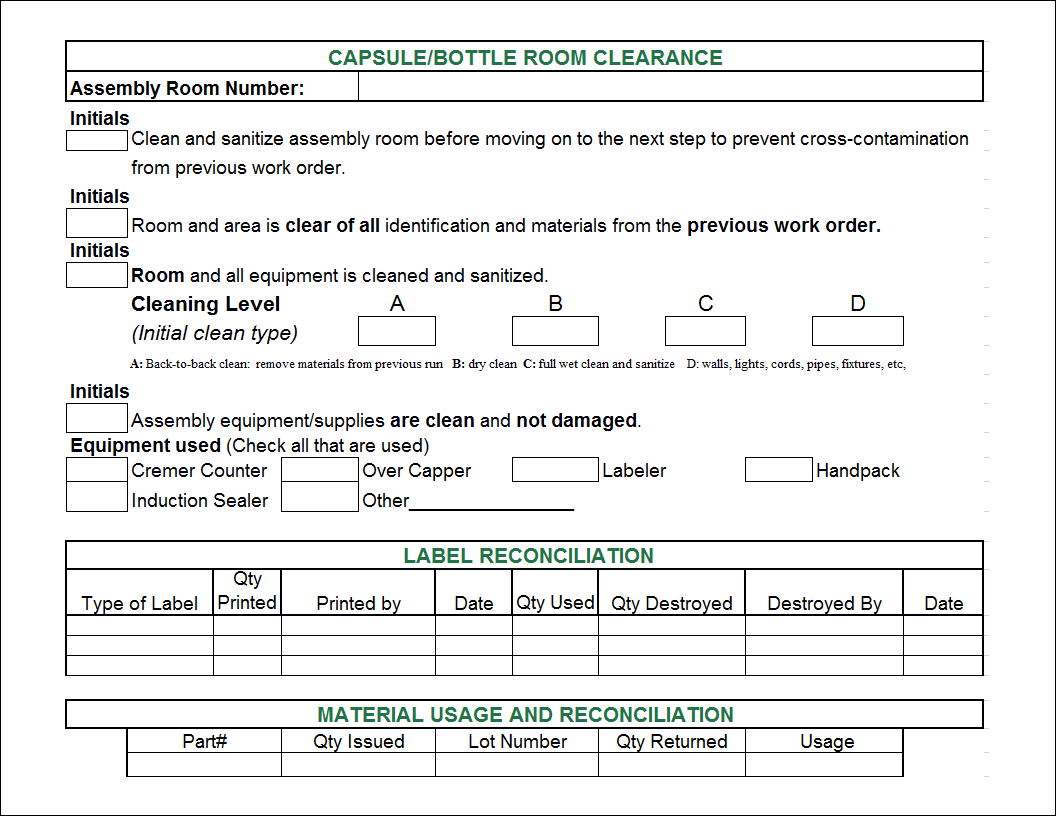
Batch Record Template prntbl concejomunicipaldechinu gov co

Design History File (DHF) vs Device Master Record (DMR) vs Device

Design History File (DHF) vs Device Master Record (DMR) vs Device

Turn On Or Off Device And Search History In Windows 10

Turn On Or Off Device And Search History In Windows 10
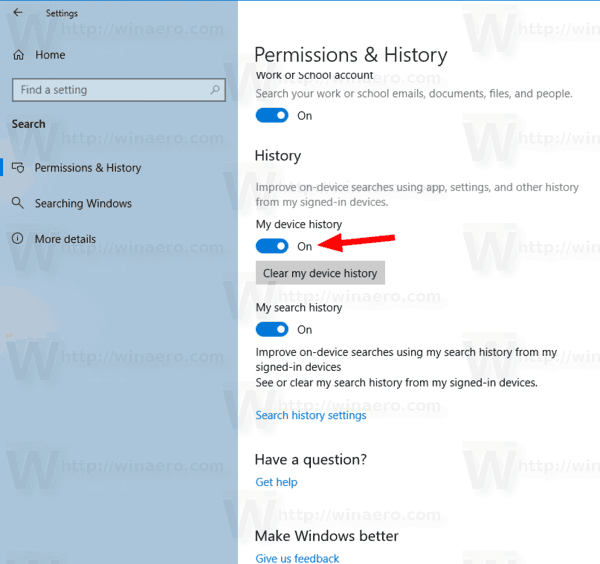
Turn On Or Off Device And Search History In Windows 10
.png)
Medical Record Template Example Free PDF Download

PPT FDA Medical Device Quality System Introduction PowerPoint
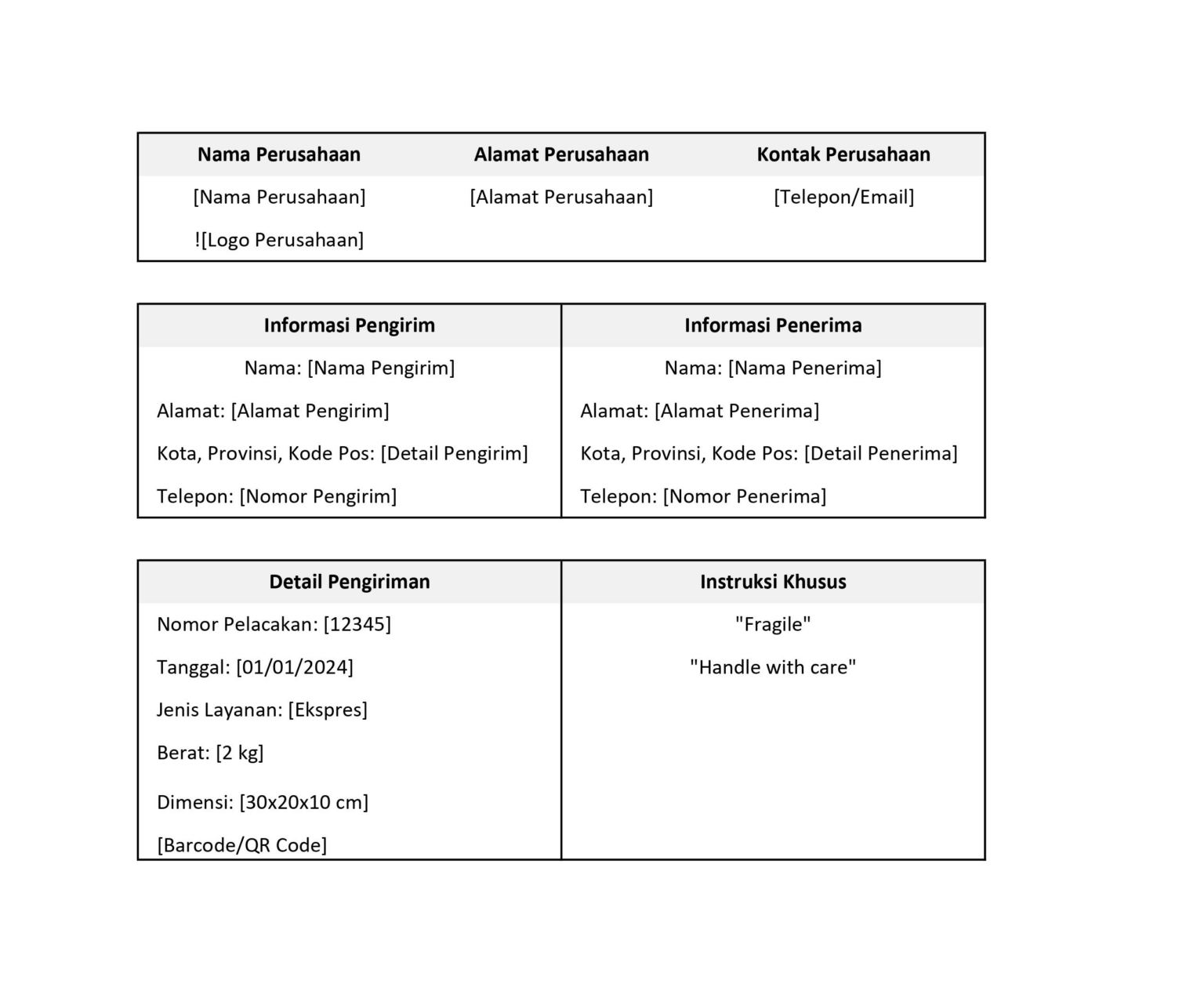
Unduh Shipping Label Template Gratis Di Sini Plugin Ongkos Kirim

Device History Record vs Device Master Record l 21 CFR 820 DHR DMR l
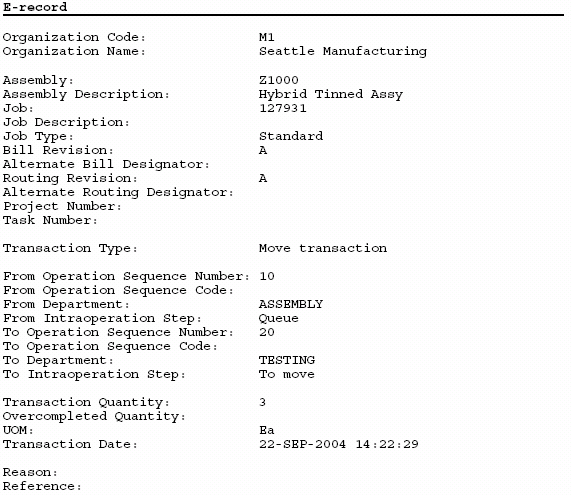
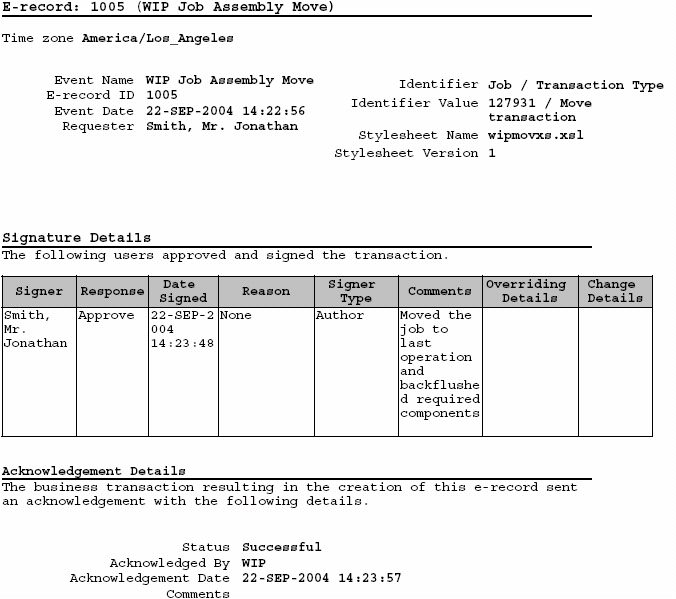
Oracle Manufacturing Implementing Oracle E Records in Discrete
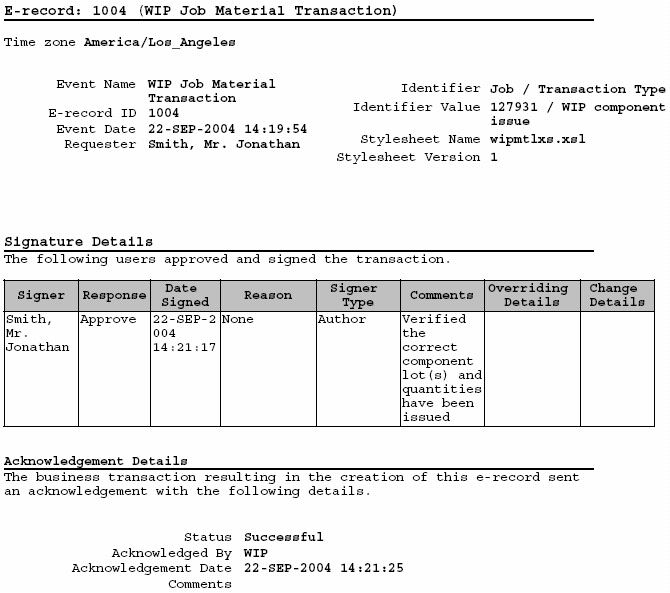
Oracle Manufacturing Implementing Oracle E Records in Discrete

PPT Design documentation PowerPoint Presentation free download ID

CSA vs CSV: FDA s New Guidance for Software Assurance Critical

CSA vs CSV: FDA s New Guidance for Software Assurance Critical